![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
The major type of reactions that alkanes undergo is: |
free radical addition reactions. |
|
|
Which of the following is the initiation step for the monobromination of cyclohexane? |
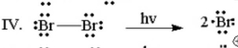
|
|
|
Which of the following is the rate-determining step for the monobromination of cyclohexane? |
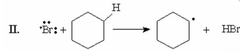
|
|
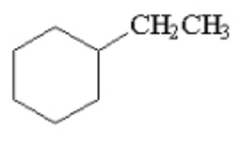
How many products are formed from the monochlorination of ethylcyclohexane? Ignore stereoisomers. |
6 |
|
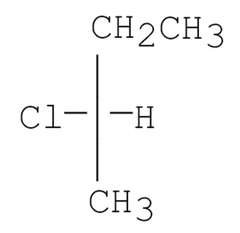
How many dichlorinated products, including stereoisomers, can be isolated when(S)-2-chlorobutane reacts with Cl2/hv? |
5 |
|
|
The reaction Br2+ CH3Br ---> CH2Br2+ HBr was carried out. Which of the following mechanism steps is both productive and relatively likely to occur? |
Br·+ CH3Br ---> HBr+· CH2Br |
|
|
Which of the following reactions is a termination step in the free radical chlorination of methane? |
· CH3+ Cl· ---> CH3Cl |
|
|
Which of the following is a chain propagation step in the free radical chlorination of methane? |
CH4+ Cl· ---> CH3+ HCl |
|
|
How many distinct dichlorination products can result when isobutane is subjected to free radicalchlorination? |
3 |
|

Which of the following is the most stable radical? |
V |
|
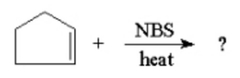
What is the major product of the following reaction? |
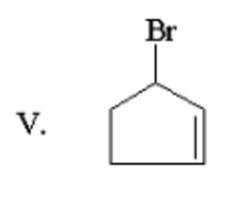
|
|
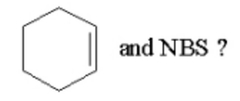
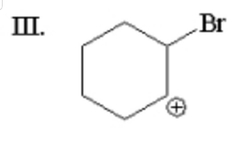
Which of the following is not an intermediate or product in the reaction of |
|
|
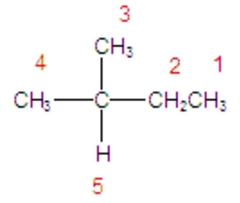
Identify the hydrogen that will react the fastest in a radical halogenation reaction. |
hydrogen 5 |
|
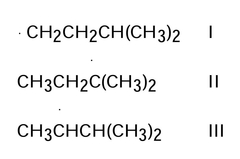
Rank the free radicals (I-III) shown below in order of decreasing stability (ie, from most stable to least stable). |
II > III > I |
|
|
Which of the halogens below undergoes free radical halogenation with ethane most rapidly? |
fluorine |
|
|
In the free radical chlorination of ethane, the step in which the Cl radical abstracts a H atom from ethane is ________ and the transition state most closely resembles ________. |
exothermic, the reactants |
|

What is the major product of the following reaction? |

|
|
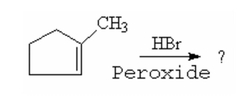
What is the major product of the following reaction? |
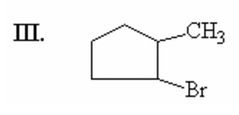
|
|

When (R)-2-bromobutane reacts with Cl2/hv, which of the following is true? |
I and II are formed in unequal amounts. |
|
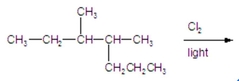
Calculate the percentage of 1-chloro-3,4-dimethylheptane formed in the following reaction. |
6.70 |

