![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
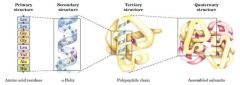
Primary ------ Secondary ------- Tert ----- -Quart
|
Sequence ---- Domain ------- Folding --------- Multi-subunit
|
|
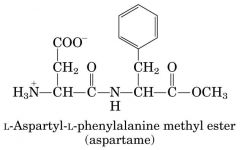
Nutrisweet
|
Primary structure --- poisonous in large amount --> gain weight taste good (sweet) eat more
|
|
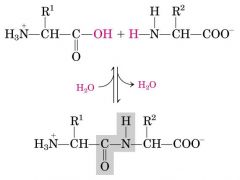
Psy bond --- Pi bond (share e O and N) ---> peptide bond
|
Al C can rotate (connector) --- Pi bond can't rotate (partial double)
|
|
|
Secondary Structure - H bond - Gly - Pro
|
alpha helix ----- beta sheet --- beta bend --> Proline (cis-trans isomer help rotate at the turn) Glycine (smallest most flexible) --- H bond -- water salt bridge
|
|
|
Forces that hold tert - quart
|
salt bridge -- H bond --- Disulfate bridge -- Hydrophobic interaction --
|
|
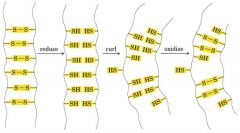
{Curly hair ---- Disulfate bridge to H bond
|
S-S (disulfate bond stable) ----- reduce to SH-HS (H bond less stable) ----- curl ----- oxidize some back S-S (more stable)
|
|
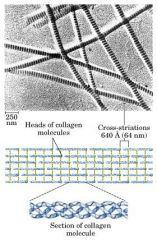
Collagen triple helix
|
Cross linked add strength to fibrous protein -- stronger than steel
|
|
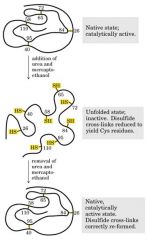
Protein denaturaiton
|
heat up - protein open - fall apart - absorption more light - increase visible
|
|
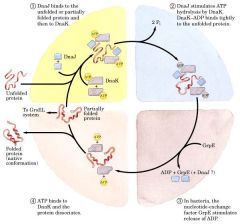
Prion --- Heat Shock Protein Hsp 70
|
chaperone help other protein to fold in a more stable protein - beta sheet - Protein is spontaneous fold with 30% help of chaperone (stablize protein during stress - temp)
|

