![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
|
What are the non-covalent forces that stabilise protein structure? |
1. Hydrogen bonds 2. Hydrophobic effect 3. Ionic interactions 4. Van der Waals forces |
|
|
What type of bond are disulphide bridges between proteins? |
Covalent bonds |
|
|
How come there is an increase in entropy when a protein folds due to the hydrophobic effect? |
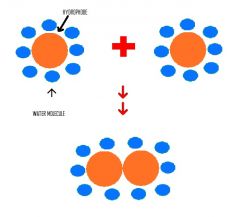
The entropy of water, not the protein increases. This is because when the protein is in its unfolded state, it forces water molecules to form highly ordered structures. However when the protein fold, water molecule are less ordered . |
|
|
In the unfolded state of a protein, are there any VdW interactions? |
No |
|
|
Where are the hydrogens bonds present in a proteins? |
Between side chains (so within the chain) of alpha helices and B pleated sheets. e.g N-H ---- O=C |
|
|
Can water molecules that are in contact with a hydrophobic side-chain form any types of covalent bond with that side chain? |
No. So they form tighter bonds with each other. |
|
|
Why do hydrogen bonds not contribute 3kcal/mol to the free energy of folding? |
No-only 1 kcsl/mol because they can also form (weak) H-bonds with water molecules in the unfolded state. When NH2 and COOH groups aren't together, they form H bonds with water. The hydrogen bond that NH2 makes on its own with water is about 1kcal/mol The hydrogen bond that COOH makes with water is about 1kcal/mol. 3-1-1 =1 kcal/mol |
|
|
Do electrostatic interactions each contribute 5kcal/mol to the free energy of folding? Why? How does the entropy of water change? |
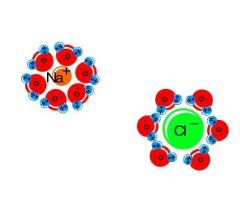
No-because the charges are fully solvated (neutralised) by water in the unfolded state. The entropic contribution of 1kcal/mol remains. When the charges are brought together, they are neutralised by each other rather than by water molecules. The entropy of water increases when they are brought together |
|
|
Entropy can be related to the number of states, or degrees of freedom of a system. This is the entry equation S=klnW What does the k stand for and what does the W stand for? |
k stands for the Bolzmann's constant W stands for the number of states |
|
|
How many conformations does the native state of a protein have? |
1 ( this is the folded state) |
|
|
For a mole of protein, how can we calculate the ΔS of folding? |
ΔS folding = S(native) - S (unfolded) |
|
|
What is the entropy of folding for a protein that has 100 residues and for every residue there are 10 conformations? |
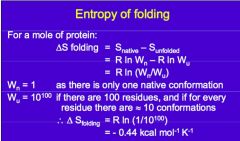
|
|

What does this show? |
The energy of non-covalent bonds (just) enough to outweigh the positive entropy of chain folding |
|
|
Why favours protein folding? |
The enthalpy of each hydrogen bond and the other non covalent forces favour folding |
|
|
What favours protein unfolding? |
Entropy of the polypeptide chain greatly favours unfolding |
|
|
The free energy of folding a protein is typically around (-) 10kcal/mol. What does this suggest? |
Proteins are very easily destabilised e.g by mutation or by a change in their environment |
|
|
What does the hydrophobic effect drive? |
Protein-protein interactions |
|
|
What is the principle driving force for protein folding (or protein-protein interactions) in aqueous solution? |
The increased entropy of the water molecules that results from burying hydrophobic amino-acid side chains. |
|
|
Is protein folding fast or slow? |
Fast |
|
|
Is protein folding spontaneous or induced? |
Spontaneous |
|
|
Does protein folding follow a defined pathway or is it trial and error? |
Defined pathways |
|
|
What does the amino acid sequence encode? |
Protein structure and folding pathway |
|
|
Describe the typical stages in going from a protein in the unfolded state, to a protein in the folded state. |
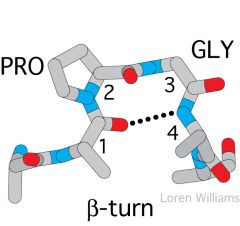
1. B turns form 2. Alpha helices form 3. B sheet form 4. Protein folds |
|
|
In vivo, what assists with protein folding? |
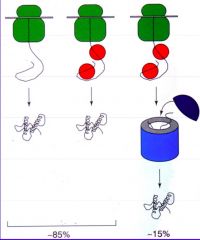
Chaperone proteins. They pick up the unfolded protein and enclose it in an isolated box that provides an environment favourable to protein folding, and then let it out once this is achieved. |
|
|
In the closed state, what surfaces are exposed on the chaperone protein? |
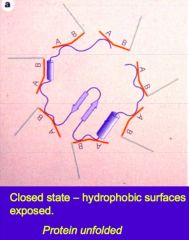
Hydrophobic surfaces |
|
|
In the open state, what surfaces are exposed on the chaperone proteins and what surfaces are hidden? |
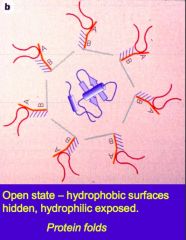
Hydrophilic residues exposed Hydrophobic surfaces hidden |
|
|
In sickle cell anaemia, which amino acid mutates (to what) and what does this cause? |
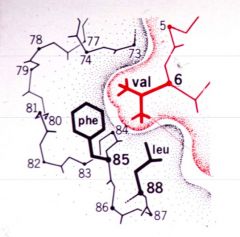
Glutamate (glutamic acid) goes to valine. This causes proteins to aggregate. |
|
|
Diseases can be transmitted through a protein. An example of this is the PrP protein. The protein exits in two possible conformations. What two forms does this protein exist in? |
The normal cellular form ( PrP c) which is benign The pathogenic form (Prp Sc) which is disease causing |
|
|
What happens when the normal cellular form of PrP is converted to the pathogenic form? |
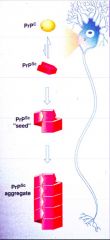
It aggregates |
|
|
What is the differences between PrP c and PrP Sc? |
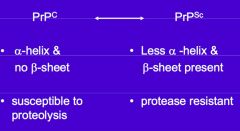
PrP c cannot polymerise but PrP Sc can |
|
|
What do the prion proteins have in common? |
Identical amino acid sequences and are coded for by the same gene |
|
|
As the polypeptide chain is synthesised, what do sections of the polypeptide rapidly assume? |
Their secondary structure |
|
|
What is an example of a disease in humans caused by protein misfolding? |
Creutzfeldt-Jakob disease (CJD) Alzheimers |
|

What are amyloids and what diseases are they associated with? |
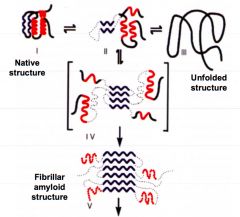
B strand rich rich aggregates They cause cell and tissue destruction Alzheimers disease CJD |
|
|
As the polypeptide is synthesised, what happens? |
Sections of the polypeptide rapidly assume their secondary structure |
|
|
Chaperones enclose the unfolded protein in an environment that is favourable for protein folding, and then let it out once it is activated. True or false. |
True |
|
|
Can chaperones give directions on how a protein folds? |
No. Chaperones simply make it more likely the the protein will fold according to its amino acid sequence by stopping it interacting with other folded proteins. |
|
|
In the alpha-helix, the length of a hydrogen bond, measured from the donor N atom to theacceptor O atom is: |
0.28 nm |

