![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
All naturally occuring amino acids are in which form? L or D isomer form? |
In the L form |
|
|
Can the C-N peptide bond (amide linkage) rotate? |
no- it is fixed, no rotation about the peptide bond. |
|
|
Which angles can rotate? |
The psi (bond between the alpha carbon and carboxyl group) and phi (bond between the nitrogen and alpha carbon) angles. |
|
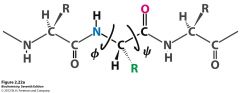
phi= between nitrogen and alpha carbon psi= between alpha carbon and carboxyl group)- fork |
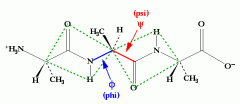
flexibility in the peptide chain is due to the phi and psi bonds. Proteins fold in the tertiary structure and so bonds need to rotate. But the peptide bond cannot rotate. |
|
|
The structure of proteins is built on 4 levels. These hierarchical levels are linked with the nature of one level influencing the next. The first structure is known as... |
the primary structure. The specific sequence/order of amino acids (determined by the genetic code, A,T,G,C). This structure is mediated solely by peptide bonds. |
|
|
Following the primary structure is the... |
secondary structure. |
|
|
What non-covalent interactions stabilise the secondary structures? |
hydrogen bonds |
|
|
What are the two characteristic secondary structures? |
a helices and B sheets. |
|
|
list some details of the a helix structure... |
-stabilised by hydrogen bonds within the chain (H bonds around 3nm in length and release 3 Kcal mol-1 when broken) -a-helices can form fibrous proteins (keratin) and globular proteins (haemglobin) -RIGHT handed helix -hydrogen bonds between the c=o of one amino acid and the N-H group of another amino acid four residues along the chain. |
|
|
list some details of the B sheet structure... |
-form from the interaction of 2 adjacent polypeptide chains which are held in close association by hydrogen bonds between the C=O and N-H groups on the two strands. -strands running antiparallel= alinged straight -strands running parallel= hydrogen bonds at an angle |
|
|
an alpha helix... |
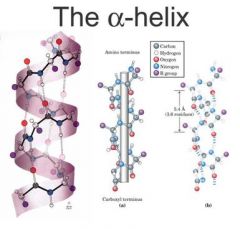
3.6 amino acids per helical turn |
|
|
an B sheet... |
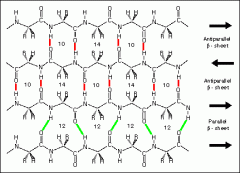
|
|
|
Another structure is a B reverse turn... |
-turn 180 degrees in every four amino acid residues -proline cannot participate in a B reverse turn -stabilised by hydrogen bonds occurring between the carbonyl oxygen of one amino acid residue and the amide of the residue 3 or 4 further along. |
|
|
following the secondary structure is the... |
super-secondary structure. These are the B barrel and the a helix bundle. |
|
|
which secondary structure is most likely to form? |
- this is influenced by the amino acid residues in the primary structure e.g glycine is particularly well suited to being located in a B turn but glycine and proline do not favour a helices- they are actually called helix disrupters. |
|
|
Following the supersecondary structure is the... |
tertiary structure. This describes the way in which the secondary structure is packed to form regions of 3D shape- the overall folded 3D structure. |
|
|
The final level of structure is the quaternary structure... |
this is the arrangement of domains or subunits. describes the way in which component subunits of a protein assemble to form the fully functioning complex. Proteins fall into 2 groups: globular and fibrous. |
|
|
What are the non-covalent forces that stabilise the folded protein structure? This is WHY protein folding takes place |
1) Hydrogen bonds 2) Electrostatic interactions 3) Van der Waals 4) Hydrophobic effect- most important driving force for protein folding NB* disulphide bonds between cystine residues are COVALENT |
|
|
membranes proteins fold so that... |
hydrophobic amino acids are on the outside and hydrophilic amino acids are on the inside---> can be placed in the phospholipid bilayer. |
|
|
How does protein folding take place? |
It is not fully understood how proteins in the cell fold up so rapidly. |
|
|
compare the alpha helix and collagen triple helix... |
Alpha helix- single chain, H bonds within the chain, 3.6 amino acid residues per helical turn, right handed sequence, does not contain proline Collagen- a triple helix, 3 chains, H bonds between chains, 3 residues per turn, left handed helix, glycine is every 3rd amino acid residue, proline is found in abundance |
|
|
what is the most abundant protein in the human body? |
Collagen |
|
|
what is the hydrophobic effect? |
tendency for non-polar surfaces to interact with each other rather than water. burying non-polar surfaces in the interior of a protein creates a situation where the water molecules can bond with each other without becoming excessively ordered--> favourable. |
|
|
list some features of globular proteins |
-compact globe or ball shaped structure -hydrophobic and hydrophilic parts -hydrophobic parts turn to the inside of the protein therefore globular proteins tend to be water-soluble -usually have metabolic roles: antibodies enzymes and haemoglobin |
|
|
list some features of fibrous proteins |
-form fibres made up of regular, repeating sequences of amino acids -tend to be water insoluble -usually have structural roles e.g collagen and keratin |
|
|
In membrane proteins the hydrophobic amino acids are on the outside and hydrophilic amino acids are on the inside. Whereas in non-membrane proteins there is a generalisation that... |
hydrophobic amino acids are on the inside and hydrophilic amino acids are on the outside. (opposite of membrane proteins) |

