![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Draw the aldehyde functional group
|
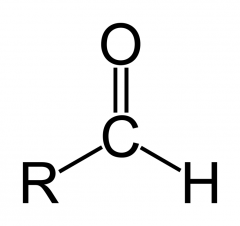
|
|
|
Draw the ketone functional group
|
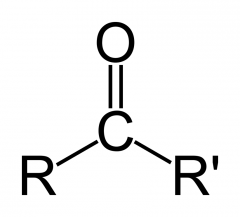
|
|
|
Draw the carboxylic acid functional group
|
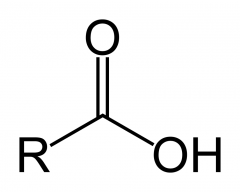
|
|
|
Draw the ether functional group
|
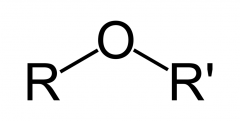
|
|
|
Explain the term elimination reaction
|
A reaction in which a small molecule is removed from a larger molecule leaving an unsaturated molecule
|
|
|
What are the products in the dehydration of alcohol?
|
An unsaturated (alkene) molecule and water
|
|
|
What conditions are needed for the dehydration of an alcohol?
|
Alcohol vapour passed over alumina catalyst at 300ºC
OR heating under reflux with concentrated sulfuric acid |
|
|
Define an addition reaction
|
A reaction where two or more molecules react to form a single larger molecule
|
|
|
Define an electrophile
|
A positive ion or molecule with a partial positive charge that will be attracted to a negatively charged region and react by accepting a lone pair of electrons to form a covalent bond
|
|
|
State the colour change that occurs when bromine reacts with an alkene
|
orange-brown to colourless
|
|
|
Draw the electrophilic reaction between bromine and ethene and explain in clear steps what has occurred
|
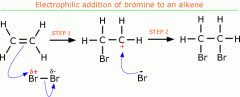
-electrons in bromine repelled by alkene bond
-bromine = polarised -δ+ bromine acts as electrophile and accepts lone pair of electrons to form covalent bond w. carbon -carbocation forms bc. lost electrons -lone pair of Br- electrons given to carbocation to form new Br-C covalent bond |
|
|
Draw the electrophilic reaction between hydrogen bromide and an ethene and state what conditions are needed
|
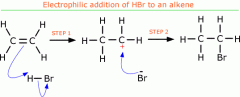
-room temperature with HBr solution in a polar solvent
|
|
|
Draw the reaction of ethene and hydrogen and state the conditions needed
|
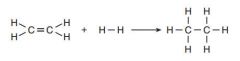
-Platinum catalyst at room temperature and pressure
OR -Nickel catalyst, finely milled, reactants heated to 150ºC |
|
|
How is ethanol made in the industry? Draw an equation for the addition reaction.
|
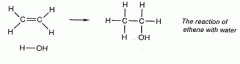
-Catalyst = phosphoric acid adsorbed onto solid silica
-300 degrees, 60 atm -water = steam |
|
|
How is ethanol made in the lab? Draw an equation for this addition reaction
|
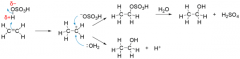
-first add concentrated sulfuric acid
-then dilute it with water -water + alkene is e.g. of hydration reaction |

