![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
|
periodic motion |
motion that is fixed around a point of stable equilibrium ex. the swinging pendulum of a grandfather clock |
|
|
simple harmonic motion |
example of periodic motion some physical quantity (x,t) that varies sinusoidally The horizontal and vertical components of the motion of an object in circular motion at constant speed are examples of SHM |
|
|
hooke' law |
the force exerted by a spring increases with increasing displacement from equilibrium mathematically F=-kx |
|
|
restoring force |
force exerted by spring on the object always towards the point of equilibrium |
|
|
transverse waves |
the displacement is at a right angle at to the direction of propagation ex. electromagnetic waves |
|
|
longitudinal waves |
the displacement is in the same direction as the direction of propagation of the wave ex. sound waves |
|
|
constructive interference |
occurs when waves of the same wavelength and frequency overlap crest of one wave is overtop the crest of another you add the amplitudes of the wave to get the resultant amplitude said to be in phase |
|
|
destructive interference |
occurs when two waves of the same wavelength and frequency overlap but are misaligned crest of one wave is in line with the trough of another resultant wave has an amplitude of 0 said to be out of phase |
|
|
beats |
when two waves of different frequency overlap beats are observed when the crests of both waves overlap and increase in amplitude is observed fb= |f1 - f2| |
|
|
standing wave |
when two waves of same wavelength and speed but travelling in opposite direction superimpose, a standing wave is formed also called a stationary wave, does not travel in either direction but oscillates up and down |
|
|
resonance |
when one object vibrating at the same natural frequency of a second object, forces that second object into vibrational motion, the two objects are said to be in resonance |
|
|
doppler effect |
the apparent change of pitch of a sound due to relative motion between the sound source and the observer applies to sound and electromagnetic waves used to find velocities of fluids |
|
|
pascal's principle |
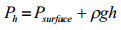
the pressure applied to an enclosed fluid is transmitted undiminished to every part of the fluid as well as the walls of the container
|
|
|
monometer |
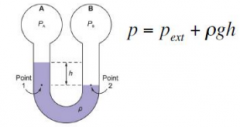
Bulbs A and B both contain gases at different pressures. They are connected by a U shaped tube which is partially filled with a fluid with density rho. Higher pressure means lower height |
|
|
buoyancy |
the force is an upward force and is constant once the object is completely submerged if the buoyant force is bigger than the force of gravity then the object floats if the buoyant force is smaller than the force of gravity the object sinks if the density of the fluid is bigger than the density of the object, the object floats |
|
|
archimedes principle |
A body immersed in a fluid experiences a vertical Apparent wt Actual wt buoyant force |
|
|
surface tension |
Property of a liquid surface defined as the force per |
|
|
capillarity |
when a glass tube is placed in water and and the water level in the tube rises ex. flow of sap in trees ex. blood flow into capillaries |
|
|
viscosity and fluid flow |
viscosity: resistance to flow ex. maple syrup is very viscous laminar flow: Layers of fluid slide smoothly past each other. Low velocities Turbulent flow : Non-laminar flow,
|
|
|
bernoulli's principle |
An increase in fluid velocity is |
|
|
zeroth law of thermodynamics |
If two systems A & B |
|
|
phase transitions |
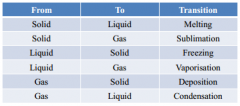
|
|
|
latent heat |
The amount of energy required to transform a Q=mL where Q is energy required, m is mass of the substance and L is the latent heat of the specific transformation of the substance at a certain temperature |
|
|
specific heat |
The amount of energy required to raise the units are joules per kilogram kelvin specific heat is equal to variable c |
|
|
conduction |
Conduction: Transfer of thermal energy from an object at high temperature to one at low temperature by contact. h is the conduction coefficient and has units of watts per meter squared kelvin ex. A cold cast iron skillet is placed onto a stovetop. When the stove is turned on, the skillet becomes very
|
|
|
convection |
transfer of thermal energy as a result of bulk motion of a fluid a heated fluid is less dense and more buoyant so it rises units are work per meter squared kelvin ex. heating water in a pot |
|
|
radiation |
transfer of thermal energy in the form of electromagnetic radiation units are work per meter squared kelvin ex. heat from the sun warming your face |
|
|
first law of thermodynamics |
based on the law of conservation of energy the change in internal energy of a system is equal to the heat added to the system minus the work done by the system U = Q - W |
|
|
charging of objects |
friction: rubbing two objects together resulting in transfer of electrons. suitable objects are determined by the electrostatic series ex. rubbing a balloon on your hair conduction: charging by contact. ex touching a positively charged rod of metal to a neutral rod of metal induction: two neutral objects in contact placed near a charged object. the object farther away gets the charge of the charged object and the closer object gets the opposite charge
|
|
|
coulomb's law |
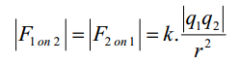
electrostatic force between two objects is directly proportional to the magnitude of each charge and inversely proportional to the distance between them |
|
|
electric field |
region around a charge where its influence is felt units in newtons per coulomb positive field lines go away from the source negative field lines go towards the source
|
|
|
electric potential |
measure of electric potential energy a charge would have if it were placed at a point does not depend on magnitude charge but its position away from reference point units are in volts which equal one joule per coulombs
|
|
|
drift velocity |
the velocity a charge has due to an electric field ex. the velocity of an electron in an electric field
|
|
|
ohm's law |
the potential difference across the material is proportional to the current potential difference is equal to the current times the resistance resistance is opposition to current flow and is given in ohms
|
|
|
kirchoff's law |
law of voltages: the algebraic sum of voltages in a circuit is zero
law of currents: at any junction the sum of currents flowing into the junction is equal to the sum of currents flowing out of the junction |
|
|
time constant |
defines the time for decays of current in a circuit the time taken for the voltage of a discharging capacitor to drop to 37% its original charge |
|
|
types of magnetic materials |
diamagnetic: weakly repelled by strong magnets ex. gold, bismuth and zinc Paramagnetic: slight magnetic attraction. ex. oxygen, aluminum, wood Ferromagnetic: strong magnetic attraction ex. iron and steel |
|
|
magnetic domain |
small regions in a magnet which behave as mini bar magnets can be aligned to produce a magnetic field which disappears when unaligned |
|
|
faraday's law and lenz law |
faraday's law: charging magnetic field produce induced emf lenz law: induced emf always results in a current whose magnetic field opposes the original change in flux |
|
|
electromagnetic wave |
electric and magnetic fields can produce a force on a charge an accelerating charge produces electromagnetic waves light is an electromagnetic wave
|
|
|
matter waves |
ordinary matter can have wave-like properties where the wavelength is related to its momentum |
|
|
de Broglie wavelength |
lambda is related to the momentum and not the actual size of the particle |
|
|
atomic models: thompson, rutherford and bohr |
thompson: plum- pudding model. A positive sphere with embedded electrons. electrically neutral rutherford: alpha scattering experiment. atom is an open space with all positive charge in the middle called the nucleus. and electrons circle the nucleus. bohr: an electron can only have those orbits in which its angular momentum in quantized, an electron gains or loses energy equal to the difference in energy between the two levels it jumps from
|
|
|
quantum numbers |
principle quantum number(n): specifies the energy shell. n=1,2,3, K,L,M. each shell has 2n^2 e- azimuthal (l): the orbital angular momentum. L= n-1, L=1,2,3,4,... s, p, d, f magnetic (m1): the spacial orientations of a subshell. values range from -1 to +1, 2l+1possibilities spin (ms): the intrinsic angular momentum. = -1/2 or +1/2 |
|
|
characteristic x ray |
radiations produced as a result of electronic transitions between certain energy states an incident electron ionizes the sample atom by ejecting an electron from an inner shell each element has a discrete set of x-ray energies named based on the lower energy state onto which the transition takes place
|
|
|
bremsstrahlung |
also called continuous x-ray and braking radiation when electron decelerates it loses energy high energy electron beam hits high Z-material which produces x-rays and heat the energy of the photon can have up to as much energy as the incident electron |
|
|
x ray production |
l |
|
|
isotope, isobar, isotone, isomer and examples |
isotope: same element, different number of neutrons ex. C- 12, 13 and 14 isobar: same atomic mass, different # of electrons ex. sulfur and chlorine both have atomic mass 40 isotone: same # of neutrons, different number of electrons ex. B with 12 for mass and 5 electrons and C with 13 for mass and 6 electrons isomer: same atomic mass and number of electrons but different energy states ex. Ze meta stable and normal Ze |
|
|
mass defect and binding energy |
mass defect: the difference between the actual mass of the nucleus and the mass of the protons and neutrons binding energy: the difference of mass energy of the nucleus and its nucleons E=mc^2 more binding energy=more energy to dissociate the nucleus=more stable |
|
|
nuclear fission and fusion |
fission: breaking nucleus into smaller parts spontaneous fission: alpha decay stimulated fission: neutron bombardment fusion: two nuclei fuse together occurs in stars like the sun resultant nucleus has bigger binding energy than starting nucleus |
|
|
alpha, beta and gamma decay |
alpha decay: emission of 2 neutrons and 2 protons in a spontaneous fission process; daughter nuclei emitted is often unstable beta decay: daughter nucleus decays by gamma or beta decay; beta-: neutron decays to a proton b- is electron-like with no mass. beta+: proton decays to a neutron and emits beta+ and a neutrino. electron capture is opposite of beta- decay gamma: photons emitted when nucleus is in excited state. decays to ground state. much higher energy than the other two |
|
|
activity |
the number of radioactive units decaying per second SI units in becquerel (Bq) Ci = 3.7 X 10^10 Bq Ci is curie A= time constant x number of nuclei |
|
|
half life and disintegration constant |
the amount of time it takes a sample to decay to half its original size half life = ln2/ decay constant |
|
|
attenuation coefficients |
total linear attenuation coefficient (μ) : units are /m; it is the logarithmic value of fractional reduction in intensity of the beam per unit thickness of the medium;depends on the density mass attenuation coefficient (μ/ρ): units are m^2/kg |
|
|
photoelectric effect, compton effect and pair production |
photoelectric effect: incoming photon is completely absorbed by the electron; part of the energy is used to overcome the binding potential and the rest is part of the 'photoelectron' compton effect: incoming photon interacts with a nearly free electron and gives the electron energy as a result a compton electron and compton scarttering photon are generated pair production: photon of very high energy converts into electron- positron pair and the process occurs in a nuclear field. the energy of the photon must be over 1.022 MeV |
|
|
exposure, absorbed dose, equivalent dose and effective dose |
exposure: defined in terms of ionization produced in the air; units in Roentgen (R); 1R is the amount of X or gamma radiation that produces ionization resulting in 1 esu of charge in 1 cm cubed of dry air; 1R = 2.58 x 10^-4 C/kg absorbed dose: energy absorbed per unit mass from any kind of radiationin any target; units in Gray (Gy); 1R = 8.8 mGy in air and 9.6 mGy in soft tissue; old units were rad and 1 rad = 0.01 Gy equivalent dose: takes into account the relative biological effects due to different radiation types; radiation weighting factor(wr) is because each type of radiation has different biological effects on the tissue; wR is 1 for xrays, gamma rays, beta particles, 5 for protons and between 5-20 for neutrons depending on their energy; it is equal to the absorbed does times the weighting factor; SI units in Sievert (Sv) and 1 REM is equal to 0.01 Sv effective dose: takes into account the different sensitivities of different tissues to incoming radiation; tissue weighting factor (wT): each tissue responds differently to incoming radiation; it is equal to the equivalent dose times the tissue weighting factor; SI units are in Sievert (Sv) |

