![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
what is dysplasia?
|
- abnormal changes in cell size, shape and organization
|
|
|
|
Why is p53 considered a proto-oncogene?
|
it is a benign cellular gene that upon mutation or disruption of normal function results in a protein that can contribute to development of a transformed cellular phenotype (unregulated cell growth or cancer)
|
|
|
|
What's the difference between necrosis and apoptosis?
|
Necrotic cell death is a pathological form of cell death resulting from acute cellular injury which is typified by rapid cell swelling and lysis
Apoptosis is controlled autodigestion by activation of endogenous proteases resulting in cell shrinkage, membrane blebbing and nuclear condensation. This results in DNA fragmentation and DNA “ladder” formation |
|
|
|
Which type of hyperplasia?
-Involved in regenerative capacity of liver |
compensatory hyperplasia
|
|
|
|
Which type of hyperplasia?
increase in uterus and mammary tissue in pregnancy |
hormonal hyperplasia
|
|
|
|
Which type of hyperplasia?
-Edndometriosis is common |
Pathologic hyperplasia
(Endometriosis: the presence and growth of functioning endometrial tissue in places other than the uterus that often results in severe pain and infertility ) |
|
|
|
What is the name of this transcription factor?
-Regulates cell cycle, functioning as a tumor suppressor. "guardians of the chromosome." |
p53
|
|
|
|
__(1) causes cells with DNA damage to undergo apoptosis, preventing proliferation of mutated cells.
|
(1) p53
|
|
|
|
Lethal cell injury or accidental cell death in the living organism. Pathological form of cell death resulting from acute cellular injury which is typified by rapid cell swelling and lysis
|
Necrosis
|
|
|
|
Programmed cell death. Control autodigestion by activation of endogenous proteases resulting in cell shrinkage, membrane blebbing and nuclear condensation. This results in DNA fragmentation and DNA "ladder" formation
|
Apoptosis
|
|
|
|
(T/F) apoptosis is energy dependant
|
True. Apoptosis is energy dependent, metabolically active
|
|
|
|
is necrosis regulated by intracellular signals
|
No, necrosis is NOT regulated by intracellular signals
|
|
|
|
What primarily results from a breakdown of fluid homeostasis?
|
Necrosis
|
|
|
|
Which type of Necrosis?
-arises from protein denaturation aggregation, commonly associated with hypoxia due to ischemic (reduced blood perfusion) injury |
Coagulative Necrosis
|
|
|
|
Which type of Necrosis?
-Often associated with tissues with little connective tissue, brain, release of hydrolytic enzymes extensively degrades lipids and proteins leading to loss of structure, also results from severe bacterial infection of any tissue |
Liquefactive necrosis
|
|
|
|
Which type of Necrosis?
-Associated with pulmonary infection, clumped cell debris due to lack of complete digestion |
Caseous necrosis
|
|
|
|
Which type of necrosis?
-Limited to breast, pancreas and abdominal fat due to excess lipolysis and subsequent saponification and formation of lipid micelles |
Fat Necrosis
|
|
|
|
What makes a cell decide to commit suicide?
|
-withdrawal of positive signals needed for continued survival.
loss of growth factor support or cell-cell interactions. -increased levels of oxidants within the cell. -damage to DNA -accumulation of proteins that failed to fold -molecules that bind to specific receptors on the cell surface and signal the cell to begin the apoptosis program. |
|
|
|
Describe the two main apoptotic signaling pathways. include the following terms: Initiation-execution; caspaces; BID.
|
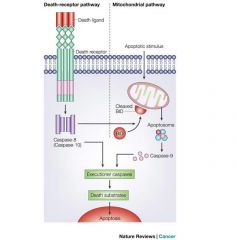
Apoptosis can be initiated by two alternative
pathways: (1) Either through death receptors on the cell surface (extrinsic pathway) or (2) through mitochondria (intrinsic pathway). In both pathways, induction of apoptosis leads to activation of an initiator caspase: caspase-8 and possibly caspase-10 for the extrinsic pathway; and caspase-9, which is activated at the apoptosome, for the intrinsic pathway. The initiator caspases then activate executioner caspases. Active executioner caspases cleave the death substrates, which eventually results in apoptosis. There is crosstalk between these two pathways. For example, cleavage of the BCL2-family member BID by caspase-8 activates the mitochondrial pathway after apoptosis induction through death receptors, and can be used to amplify the apoptotic signal. |
|
|
|
As a biochemical marker of Apoptosis, what happens during the INDUCTION OF THE MITOCHONDRIAL PERMEABILITY TRANSITION?
|
a) loss of mitochondrial inner membrane potential.
b) release of cytochrome c |
|
|
|
As a biochemical marker of Apoptosis, what happens during the ACTIVATION OF PROTEASE CASCADE?
|
a) members of interleukin-1ß converting enzyme(ICE) family of proteases, these proteases called CASPACES, family of cysteine proteases which cleave after aspartate residues, i.e. DEVD, YVAD)
b) granzyme B c) calpain |
|
|
|
What kind of drug would would cause the following effects in cellular processes:
1. This kind of drug disrupts DNA structure. 2. Disrupts cell division. 3. Affect nucleotide synthesis. 4. disrupt DNA uncoiling |
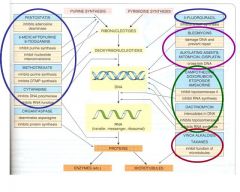
1. DNA Alkylating Agents
2. Microtubule Stabilizers 3. Antimetabolites 4. Topoisomerase Inhibitors |
|
|
|
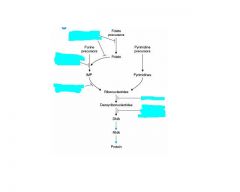
Antimetabolites:
-Explain the different ways antimetabolites can inhibit nucleotide synthesis. |
1. Inhibitors of folate metabolism
2. Inhibitors of purine synthesis and metabolism.(block de novo synthesis or conversion from salvage pathway). 3. inhibitors of ribonucleotide reductase(blocks synthesis of deoxyribonucleotides) 4. inhibitors that are incorporated into DNA. |
Analogs of folate, purines, and pyrimidines that inhibit enzymes involved in nucleotide synthesis
|
|
Match the following Anti-Metabolite Drug classes with diagram:
-Sulfonamide DHFR inhibitors - 6-Mercaptopurine Thioguanine(2 locations) - Hydroxyurea Fludarabine, Cytarabine, and Cladribine |
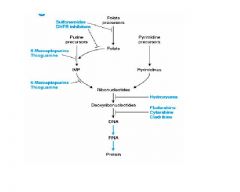
Harder question, but it's a hard test
|
|
|
|
requirement for nucleotides met by both dietary intake or ___(1)
|
(1) de novo synthesis
|
|
|
|
The salvage pathways are a major source of nucleotides for synthesis of DNA, RNA, and __(1)
|
(1) enzyme co-factors
|
|
|
|
if the nucleosides and/or bases are not re-utilized the purine bases are further degraded to __(1) and the pyrimidines to __(2) and __(3), NH3 and CO2
|
if the nucleosides and/or bases are not re-utilized the purine bases are further degraded to uric acid(1) (urate crystals) and the pyrimidines to b-alanine(2) and b- aminoisobutyrate(3), NH3 and CO2
|
|
|
|
Allopurinol inhibits __(1)
|
Xanthine oxidase
|
|
|
|
What enzyme salvages guanine and hypoxanthine
|
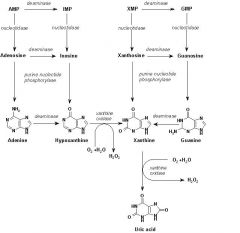
HGPRTase(if insufficient amount is salvaged, gout development ensues)
|
|
|
|
Folic acid is critical for what step in de novo purine synthesis?
|
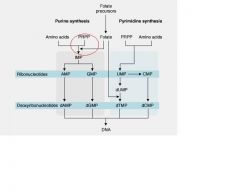
Essential cofactor in the synthesis of inosine monophosphate(IMP)
|
|
|
|
Folic acid is critical in what part of pyrimidine de novo synthesis?
|
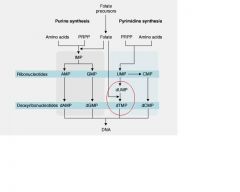
Folate required for the methylation of deoxyuridylate(dUMP)
|
|
|
|
major site of purine synthesis is...
|
Liver
|
|
|
|
___(1) is a rate limiting step, first step of De Novo Purine synthesis. Feedback inhibited by end products of de novo synthesis, AMP and/or GMP.
-Inhibited by ___(2) metabolite |
(1) PRPP synthetase
(2) Mecaptopurine |
|
|
|
purine base is built upon the ribose by __(1) and __(2) reactions.
|
purine base is built upon the ribose by aminotransferase(1) and transformylation(2) reactions.
|
|
|
|
__(1) is necessary to turn Dihydrofolate into Tetrahydrofolate. What drugs block this pathway?
|
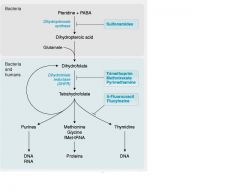
(1) DHFR(Dihydrofolate reductase)
-Inhibiting drugs: Trimethoprim, Methotrexate, Pyrimethamine |
|
|
|
This drug is selective for bacterial DHFR; bacteriostatic; often used in combination with sulfonamides to sequentially inhibit folate metabolism; since it is a reversible DHFR inhibitor, less competition, increased efficacy
|
Trimethoprim
|
|
|
|
inhibiting folate synthesis decreases substrate for __(1)
|
(1) DHFR-dihydrofolate reductase
|
|
|
|
What substance inhibits Ribonucleotide synthesis?
|
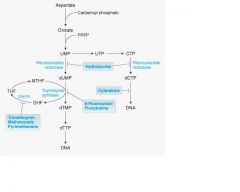
Hydroxyurea
|
it's an antineoplastic
|
|
|
What chemo drug prevents DNA synthesis' insertion?
|
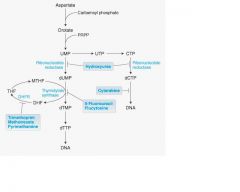
Cytarabine
|
It's a metabolite
|
|
|
__(1) is a Non-selective inhibitor of Dihydrofolate reductases.
|
Methotrexate
|
|
|
|
__(1) is an irreversible inhibitor of thymidylate synthase and is a pro-drug.
|
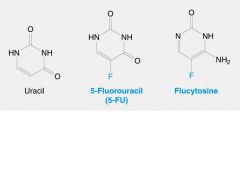
(1) 5-FU(5-Fluorouracil)
|
|
|
|
FdUMP forms a covalent ternary complex with __(1) and ___(2)
and blocks __(3) formation, dominate mechanism of anti-metabolite |
FdUMP forms a covalent ternary complex with methyleneTHF(1) and thymidylate
synthase(2) and blocks dTMP(3) formation, dominate mechanism of anti-metabolite |
|
|
|
hydroxyurea block reduction of the __(1) to __(2)
|
(1) ribose
(2) deoxyribose -also used in treatment of sickle cell anemia. |
|
|
|
What four drugs inhibit inosine monophosphate dehydrogenase? (dehdrogenase responsible for changing IMP to XMP during purine synthesis)
|
(1)6-mercaptopurine
(2) Azathioprine(AZA) (3) Thioguanine (4) Mycophenolic acid First two can also inhibit Adenylsuccinate sunthetase |
|
|
|
__(1) is a feedback inhibitor of PRPP synthase(first enzyme in de novo purine synthesis.)
|
(1) T-IMP
|
|
|
|
Allopurinol blocks degradation of __(1)
|
6-MP(Mercaptopurine). Co-administration of allopurinol lowers the dose of 6-MP required but increases the half-life and possible toxicity of the drug.
|
|
|
|
This nucleoside analog inhibits purine metabolism via Adenosine Deaminase(inhibits it).
|
Pentostatin
|
|
|
|
What happens if there is an increase in intracellular adenosine and deoxyadenosine.
|
Increases the metabolite S-adenosylmethionine which is especially toxic to lymphocytes.
|
|
|
|
What does pentostatin normally treat?
|
Hairy cell leukemia.
|
|
|
|
Converted to GHPRTase to 6-thioGMP
|
Thioguanine
|
|
|
|
-Once incorporated into DNA,
interferes with transcription and DNA replication, also decreases GMP synthesis. - Used to treat myelocytic leukemia but strongly suppresses bone marrow function and has gastrointestinal toxicity issues. |
Thioguanine
|
|
|
|
Triphosphorylated form inhibits
DNA polymerase and ribonucleotide Reductase, reduces nucleotide synthesis also can be incorporated into DNA, causes chain termination Used in treating lymphocytic leukemia and B-cell lymphomas |
Fludarabine-5-Phosphate (Fludara)
|
|
|
|
Phosphorylated by deoxycytidine kinase in cells with a high ratio of deoxycytidine kinase to deoxynucleotidase there is little deoxynucleotide deaminase in lymphocytes and monocytes,
aids conversion to active triphosphate deoxynucleotide, 2-chloro-dATP. -When incorporated into DNA causes strand breaks used in treating hairy cell leukemia both Fludara and Leustat are resistant to deamination by adenosine deaminase. |
Cladribine (Leustat)
|
|
|
|
-Metabolized to araCTP and
competes with CTP for incorporation into DNA -Results in chain termination since steric interaction of 2’-OH on ribose disrupts base stacking. |
Cytosine Arabinoside
(Cytarabine or AraC) |
|
|
|
Interferes with DNA methylation which is a critical feature in regulating gene expression.
-Both reagents used in treating myelocytic leukemias. |
5-Azacytidine
|
|

