![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
85 Cards in this Set
- Front
- Back
|
Alpha cell
1. % islet cell 2. Hormone 3. Function |
1. 20%
2. Glucagon 3. Mobilize glycogen |
|
|
Delta cell
1. % islet cell 2. Hormone 3. Function |
1. 3-5%
2. Somatostatin 3. Inhibit secretory cells |
|
|
F cell
1. % islet cell 2. Hormone 3. Function |
1. <2%
2. Pancreatic Polypeptide 3. Facilitate digestion |
|
|
Beta cell
1. % islet cell 2. Hormone 3. Function |
1. 75%
2. Insulin 3. Storage and Anabolic hormone |
|
|
Aside from Insulin, what other 2 things do pancreatic Beta cells secrete?
|
1. C-peptide
2. Islet amyloid polypeptide = amylin |
|
|
Insulin Structure:
-insulin comes from __1__ synthesized in pancreatic __2__ cells -Proinsulin is __3__ to release: __4__ & __5__ |
1. Proinsulin
2. Beta 3. hydrolyzed 4. Insulin 5. C-peptide |
|
|
Removal of __1__ forms Insulin, a small protein consisting of __2__(#) amino acids arranged in __3__(#) chains
|
1. C-peptide
2. 51 3. two |
|
|
How many amino acids does the alpha chain of Insulin contain?
Beta chain? |
Alpha = 21
Beta = 30 |
|
|
How is Insulin stored in Beta cells?
|
Insulin crystals are stored in secretory granules
|
|
|
How much Insulin does the human pancreas normally contain?
|
8 mg (200 U)
|
|
|
Explain the resting state Beta cells, when Insulin is not secreted
|
-At rest, Beta cells have low ATP
-Low ATP causes ATP-gated K+ channels to be open -K+ diffuses out to keep Beta cell depolarized (negative) -Insulin secretion is minimal |
|
|
Explain the mechanism of Insulin secretion
|
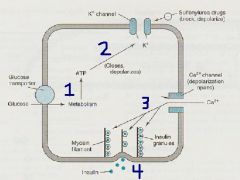
1. Stimulation by Hyperglycemia or Insulin Secretagogues
2. intracellular ATP increases → closes K+ channels 3. Depolarization of Beta cell → Ca++ chennels open 4. Ca++ enters Beta cells and increases Intracellular Ca++ → Insulin is secreted |
|
|
What is the principle stimulus of Insulin secretion?
What are some other minor stimuli? |
Glucose
Sugars, Amino acids, Secretagogues |
|
|
What are the basal values of circulating insulin detected by RA in normal subjects?
Peak levels? When are peak levels found? |
Basal = 5-15 mU/mL (30-90 pmol/L)
Peak = 60-90 mU/mL (360-540 pmol/L) = during meals **multiply times 6 to convert from mU/mL to pmol/L |
|
|
What are the 3 main sites of Insulin receptors?
|
1. Liver
2. Muscle 3. Adipose tissue |
|
|
What is the half-life of circulating Insulin?
What is the reason for this half-life? (Be specific) |
3-5 minutes
Removed by circulating Insulinase acting in: --Liver = 60% --Kidney = 35-40% |
|
|
Explain the structure of the Insulin Receptor
|
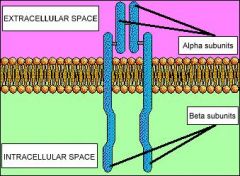
Consists of 2 Covalently linked Heterodimers each containing:
-Alpha subunit = extracellular recognition site -Beta subunit = spans cell membrane and contains Tyrosine Kinase |
|
|
Explain what happens when Insulin binds to the Insulin Receptor
|
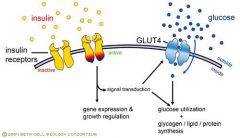
-Insulin binds to the Alpha subunit -> brings the catalytic loops of the Cytoplasmic Beta subunits closer together to facilitate Tyrosine Kinase activity
-Activated TK P's the docking proteins IRS to translate the insulin signal -Phosphorylation network causes translocation of Glucose Transporters (GLUT4) to the cell membrane -Increased glucose uptake, glycogen formation, & multiple effects of protein synthesis, lipolysis, & lipogenesis |
|
|
List the 4 main sites of Action of Insulin
|
1. Glucose transporters
2. Liver 3. Muscle 4. Adipose tissue |
|
|
Explain Insulin's effect on Glucose Transporters
Which is the most important transporter for Hypoglycemia? |
Insulin facilitates glucoses movement across cell membranes
GLUT 4 |
|
|
Explain Insulin's effects on the Liver
|
-increases storage of GLYCOGEN
-decreases postabsorptive catabolism (breakdown) |
|
|
Explain Insulin's effects on Muscle
|
promotes Protein and Glycogen synthesis
|
|
|
What are Insulin's effects on Adipose Tissue?
|
reduces free fatty acids → promotes Triglyceride storage
** = fat deposition |
|
|
What 2 Insulin effects cause Hypoglycemia?
|
1. inhibition of liver glucose production (decreases gluconeogenesis and glycogenolysis)
2. stimulates glucose uptake and metabolism in liver, adipose, skeletal muscle |
|
|
How is insulin usually administered clinically and what are onset, peak, and duration of action related to?
|
Subcutaneously
Onset, peak, & duration are DOSE-related |
|
|
Short-acting or Regular insulin mimic the __1__ rise in insulin and is given IV for __2__ or __3__
|
1. post-prandial (= the rise in insulin after meals)
2. Hyperglycemic emergencies 3. Diabetic Ketoacidosis |
|
|
Insulin preparations that mimic the basal level of insulin
|
NPH
Lente Ultralente |
|
|
Describe the administration of Insulin preparations
|
Are given as mixtures to produce desired times of onset, peak, and duration of action
Varies from patient to patient |
|
|
Ultra-long-lasting insulin that produces a sustained "peakless" absorption profile, and can provide once-daily effective 24-hour plasma levels
|
Glargine
|
|
|
List the 2 Ultra-short-acting Insulin preps and their duration
|
Lispro & Aspart
3-4 hours |
|
|
List the short acting Insulin prep and its duration
|
Regular
5-7 hours |
|
|
List the Intermediate-acting Insulin prep and duration
|
Lente or NPH
18-24 hours |
|
|
List the Long-lasting insulin prep and duration
|
Ultralente
18-28 hours |
|
|
What is the Ultra-long-acting insulin prep and duration?
|
Glargine
>24 hours |
|
|
What is the name for the Inhalable Insulin preparation?
Describe its characteristics |
Exubera
rDNA origin used as a hand-held inhaler for use in combo with oral hypoglycemic drugs or longer-acting insulin |
|
|
When is Inhalable Insulin used?
|
Before meals
**is a rapid-acting insulin powder inhaled through the mouth into the lungs |
|
|
Inhalable Insulin is as effective as __1__ insulin injections, but exact __2__ is difficult
|
1. Short-acting
2. dosing |
|
|
In what 2 situations should Exubera not be used?
|
1. Smokers = either still smoking or stopped smoking < 6 months ago
2. Patients with Chronic Lung Disease (asthma, COPD, emphysema) |
|
|
In which type of diabetes is Insulin therapy the mainstay treatment?
|
Type I diabetics
**Insulin is deficient in these patients so give Insulin |
|
|
When is Insulin treatment used in Type II Diabetics?
|
when the diabets is not adequately controlled by DIET &/or Oral Hypoglycemic drugs
**1 of 5 will need insulin therapy |
|
|
In what 2 ways does Insulin injected SubQ differ from endogenously secreted insulin?
|
1. does not reproduce the rapid rise and fall occuring when insulin is secreted due to stimulation by ingested food
2. diffuses into the systemic circulation instead of being secreted into the portal circulation (SubQ eliminates the direct hepatic effects produced by normal secretion) **these are 2 drawbacks |
|
|
What is the ideal goal in Insulin therapy?
|
Normalize blood glucose and all aspects of glucose metabolism
**this is difficult to achieve |
|
|
What is the most common complication of Insulin therapy? What things can trigger it?
|
Insulin Hypoglycemia (low blood sugar)
Delayed meals, unusual physical exertion, or insulin overdose |
|
|
What plasma level do signs of Hypoglyecmia manifest at?
|
60-80 mg/dL
|
|
|
What are the signs of Hypoglycemia?
|
1. Neuroglycopenic = CNS impairment manifested as mental confusion, bizarre behavior, convulsions, or coma
2. Autonomic hyperactivity -Sympathetic = tachycardia, palpitations,sweating, tremors -Parasympathetic = nausea & hunger |
|
|
What rapidly releives Insulin Hypoglycemia?
What should be given to unconscious patients? |
Glucose = OJ or any sugary food or drink
IV 50% glucose, 20-50 mL over 2-3 min |
|
|
Which type of Diabetes are Oral antidiabetic drugs usually used for?
|
Type II
|
|
|
List the 4 categories of Oral Hypoglycemics
|
1. Insulin Secretagogues
-Sulfonylureas -Meglitinides 2. Biguanides 3. Thiazolidinediones 4. Alpha-glucosidase inhibitors |
|
|
What is the general mechanism of the Insulin Secretagogues?
|
stimulate Insulin release by closing K+ channels in pancreatic Beta cells
|
|
|
What are the 2 groups of Insulin Secretagogues?
|
1. Sulfonylureas
2. Meglitinides -Repaglinide -Nateglinide |
|
|
Describe the mechanism of Sulfonylureas?
|
1. bind to high affinity receptors for K+ channels in pancreatic beta cells
2. inhibit K+ efflux to cause depolarization 3. Open voltage-gated Ca++ channels 4. Ca++ influx 5. Insulin release |
|
|
List 4 1st generation Sulfonylureas
|
1. Acetohexamide
2. Chlorpropamide 3. Tolazamide 4. Tolbutamide |
|
|
List the 4 2nd generation Sulfonylureas
|
1. Gli-me-piride
2. Gli-cazide 3. Gli-pizide 4. Gly-buride **all start with Gl- |
|
|
Aside from stimulating Insulin secretion, what other effect do Sulfonylureas have?
|
reduce serum Glucagon levels
|
|
|
When are Sulfonylureas mainly used?
|
Treating Type 2 diabetes whose hyperglycemia cannot be controlled by diet alone
|
|
|
What are 2 important properties of 2nd generation of Sulfonylureas?
|
1. more effective BUT cause fewer adverse effects = good quality
2. may produce dangerous hypoglycemia in diabetics who are elderly or have Cardiovascular disease = bad effect |
|
|
Which 2nd Generation Sulonylurea has the shortest half-life and is less likely to cause severe Hypoglyemia
|
Glipizide
**less likely to cause hypoglycemia b/c it has a short half-life and therefore has less time to cause tissue to uptake glucose |
|
|
Would you use 1st or 2nd generation Sulfonylureas in Elderly & CV disease patients?
|
1st generation
|
|
|
What 2 Sulfonylureas are safest for the elderly? Why?
|
Tolazamide & Tolbutamide
-relative short duration of action -severe hypoglycemic episodes are unlikely |
|
|
What are the 2 available Meglitinides?
|
Repa-glinide
Nate-glinide |
|
|
What is the main use for Meglitinides?
|
controlling postprandial Glucose levels
|
|
|
What are the sites of action of Meglitinides?
|
1. unique binding site
2. two other sites in common with Sulfonylureas |
|
|
In what type of patient should you be cautious when giving Meglitinides?
|
someone with Hepatic dysfunction
|
|
|
What is the distinction between Sulfonylurea and Meglitinides?
|
Meglitinides (Repaglinide specifically) can be used in Type II diabetics who are allergic to Sulfonylureas b/c Repaglinide structure does not contain Sulfur
|
|
|
Oral Hypoglycemic under the category of Biguanides
|
Metformin
|
|
|
Hypoglyemic drug that is referred to as "euglycemic" or antihyperglycemic b/c it does not cause hypoglycemia but reduces fasting or postprandial blood glucose levels
|
Metformin
|
|
|
List the 4 proposed mechanisms of Metformin
|
1. direct stimulation of tissue glycolysis
2. reduced hepatic gluconeogenesis 3. reduced GI glucose absorption 4. reduced plasma glucagon |
|
|
When is Metformin used? (2)
|
1. obese patients with "insulin resistance syndrome"
2. Type II diabetics who do not respond to Sulfonylureas alone |
|
|
What is the most frequent side-effect of Metformin?
|
GI effects
-anorexia, NVD, abdominal discomfort |
|
|
Under what 4 instances is Metformin contraindicated?
|
1. Renal disease
2. Hepatic disease 3. Alcoholism 4. Chronic Cardiopulmonary dysfunction |
|
|
List the 2 Thiazolidinediones (TZD's)
|
Rosi-glitazone
Pio-glitazone **-GLITAZONE |
|
|
What is the mechanism of the TZD's?
|
diminish insulin resistance by enhancing the tissue sensitivity to insulin
|
|
|
Diabetic drugs that are agonists of PPAR-gamma (peroxisome proliferator-activated receptor-gamma) nuclear receptors found in muscle, fat, and liver
|
TZD's
= Rosi-glitazone = Pio-glitazone |
|
|
What does PPAR-gamma do?
|
activates insulin-responsive genes that regulate carbohydrate and lipid metabolism (increases glucose uptake and metabolism in muscle and adipose tissue)
|
|
|
What is the major site of action of the TZD's (-glitazones) in diabetics?
What beneficial efects may this have? |
Adipose tissue
Redistribution of body fat causing reduced visceral fat mass and loss of central obesity |
|
|
Rosiglitazone and Pioglitazone are clinically effective in __1__ diabetes without producing __2__
|
1. Type II
2. Hepatotoxicity |
|
|
What are the adverse effects of TZD's? (4)
|
1. Edema
2. Anemia 3. Fluid retention 4. Weight gain |
|
|
What are the 2 Alpha-Glucosidase Inhibitors?
|
A-carb-ose
Mi-glitol |
|
|
What is the mechanism of Alpha-Glucosidase Inhibitors in treating Diabetes?
|
-inhibit intestinal alpha-glucosidases that normally facilitate digestion of starches and disaccharides into monosaccharide molecules absorbed in the duodenum and upper jejunum
-cause hypoglycemia by reducing upper intestinal digestion of starch and disaccharides to defer digestion to the distal small intestine |
|
|
What are the Alpha-Glucosidase Inhibitors used for clinically?
|
Type II diabetics prior to ingestion of the first portion of each meal
|
|
|
What are the prominent side-effects of Alpha-Glucosidase Inhibitors?
|
Flatulence, diarrhea, and abdominal pain caused by gas released by short-chain fatty acids fermented from undigested carbohydrates in the colon
|
|
|
When are Alpha-Glucosidase Inhibitors contraindicated?
|
Chronic or inflammatory bowel disease
Any intestinal condition that may be worsened by gas and distention |
|
|
Oral Antidiabetics associated with Disulfarim-effect
|
1st Generation Sulfonylurea's
-Chlorpropamide |
|
|
Anti-diabetic drug that causes Lactic Acidosis
|
Metformin
|
|
|
Pancreatic hormone product that is used as a stimulant in beta-adrenergic blocker overdose
|
Glucagon
|

