![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
114 Cards in this Set
- Front
- Back
|
What is Folate essential for?
|
DNA synthesis and mitosis of proliferating cells
|
|
|
What is the function of Folate?
|
Coenzyme for 1-carbon transfer involved in the biosynthesis of Purines and Pyrimidines
|
|
|
What are the sources of Folic Acid?
|
Plants and Animals:
Yeast Liver Kidney Green Vegetables |
|
|
Folate Absorption:
-readily and completely absorbed from the __1__ by __2__ -__3__ folate is absorbed daily -absorption is increased in __4__, but so is __5__ |
1. Small Intestine
2. Active transport system 3. 50-200 micro-grams (=10-25% of folate) 4. pregnancy 5. demand |
|
|
Describe the process of Folate absorption
|
1. Polyglutamate form is ingested
2. Glutamyl transferase in GI tract clips glutamate residues 3. Monoglutamate form is absorbed via active and passive transport in the PROXIMAL JEJUNUM |
|
|
Folate Distribution:
-__1__ and other tissues store __2__ of folate -Major dietary and storage form of folate is __3__ -Because the body stores relatively little folate (relative to high demand), __4__ anemia can develop in __5__ (timespan) following folate deficiency |
1. Liver
2. 5-20 milligram 3. 5-methyl-THF 4. Megaloblastic 5. 1-6 months (relatively fast) |
|
|
How are Folates excreted?
|
Metabolized and excreted in Urine and Feces
|
|
|
When is Folate deficiency due to inadequate dietary intake common?
|
Alcoholics
|
|
|
Give four examples of when Folate is needed in increased requirements?
|
1. Pregnancy
2. Renal dialysis -blood folates are removed -EPO is also removed 3. Proliferative disorders (CA, leukemia, etc) 4. Hemolytic anemia |
|
|
What drugs interfere with the utilization of Folate and can lead to deficiency?
|
1. Anticonvulsants
-Phenytoin -Primidone -Mephobarbital 2. Oral Contraceptives 3. Isoniazid |
|
|
What is maternal Folate deficiency associated with?
|
Neural Tube Defects = Spina bifida
|
|
|
Why is Folate sometimes used to treat Coronary Heart Disease?
|
Hyper-homocysteinemia is a possible risk factor for CHD
-Folate and B12 are BOTH needed to convert Homocysteine -> Methionine -Methionine is a major Antioxidant in the body |
|
|
What can cause low Methionine levels?
|
Both Folate and B12 can cause Methionine levels to be low, either together or independently
|
|
|
What are the 3 functions of Vitamin B12?
|
1. DNA synthesis: converts 5-methyl-THF -> THF
2. Lipid synthesis: converts Methylmalonyl-CoA -> Succinyl-CoA 3. Amino acid synthesis: B12 + Folate convert Homocysteine -> Methionine |
|
|
Which defiency can cause neurological symptoms: Folate or B12? Why?
|
B12
- converts Methylmalonyl-CoA -> Succinyl-CoA -Succinyl-CoA helps to produce Myelin |
|
|
Why does B12 have to be present for Folate's effects?
|
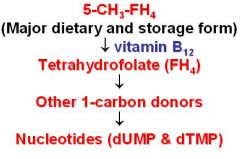
B12 converts dietary Folate (5-methyl-THF) to its active form (THF)
|
|
|
-In Vitamin B12 deficiency, levels of __1__ increase with a decrease in the other forms of folate required for __2__ synthesis
-This defect can be circumvented by administration of __3__ which can be reduced to __4__ by __5__ -Thus the defects in Nucleotide synthesis caused by B12 deficiency can be corrected by __6__ treatment |
1. 5-methyl-THF
2. nucleotide 3. Folic acid 4. THF 5. Dihydrofolate reductase 6. Folic acid |
|
|
Vitamin B12 structure:
__1__-like ring system complexed with __2__ |
1. Porphyrin
2. Cobalt |
|
|
Cobalamin (B12) forms:
-Active form: R = 1 -Drugs: R = 2 -Food: R = various ligands |
1. 5-deoxyadenosyl or Methyl group
2. Cyano (CN-) or Hydroxy (OH-) **drugs are converted to active forms in the body |
|
|
What are the sources of Vitamin B12?
|
Meat (liver)
Eggs Dairy products |
|
|
Absorption of Vitamin B12 requires what?
|
Intrinsic Factor (glycoprotein) synthesized by Parietal Cells in the Stomach
|
|
|
Where is the B12/IF complex absorbed?
|
Ileum
|
|
|
Vitamin B12 Distribution:
-transported via __1__, a plasma glycoprotein -excess is stored in the __2__ -it takes __3__ to deplete stores from the body |
1. Transcobalamin II
2. Liver 3. 3-6 years = deficiency occurs ver very slow compared to Folate (1-6 months) |
|
|
Describe the excretion of Vitamin B12
|
-occurs in Bile but undergoes Enterohepatic circulation and most is reabsorbed from the Small Intestine
-When Transcobalamin is saturated excess B12 is excreted in Urine |
|
|
Lack of Intrinsic Factor = ?
How is this treated? |
Pernicious Anemia
treat with B12 |
|
|
What causes Pernicious Anemia?
|
Autoantibodies to Parietal Cells or Intrinsic Factor
|
|
|
Aside from Pernicious Anemia, what are 3 other causes of Vitamin B12 deficiency?
|
1. Lack of receptors for IF/B12 complex in the Ileum -> genetic or surgical resection
2. Fish tapeworm infections 3. Patient with Gastrectomy -> no Parietal Cells = no IF |
|
|
Therapeutic uses of B12:
-only approved use is treatment of __1__ -usually given by __2__ |
1. B12 deficiency
2. Intramuscular injection |
|
|
T or F: Vitamin B12 can be toxic if given in large amounts
|
False: it is nontoxic even in large amounts
|
|
|
What are the 2 therapeutic preparations of Vitamin B12?
|
Cyanocobalamin
Hydroxycobalamin |
|
|
List the properties of Cyanocobalamin (3)
|
1. available nasally, orally, parenterally
2. does not cause Ab response 3. preferred agent for Long-term use |
|
|
List one advantage and one disadvantage of Hydroxycobalamin
|
Ad: highly protein bound and remains in circulation longer
Disad: some patients produce ANTIBODIES against Hydroxycobalamin-Transcobalamin II complex |
|
|
Why is it important to distinguish between Folate and B12 deficiency?
|
If it is B12 deficiency and you give Folate:
-the anemia can be corrected -BUT Neurological symptoms can still occur |
|
|
What are the 2 clinical tests to determine Folate and B12 deficiency?
|
Folate -> red cell levels
B12 -> serum levels |
|
|
How is a Schilling Test performed?
|
Oral administration of radioactive Vitamin B12 with and without pig IF, after which the presence of radioactivity in the urine is determined
|
|
|
A negative Schilling Test of BOTH free B12 and B12/IF indicates what?
|
Malabsorption in the distal ileum
-inflammatory bowel disease -small bowel resection |
|
|
A negative Schilling Test of JUST B12 indicates what?
|
Malabsorption due to the lack of Intrinsic Factor
-Gastrectomy -Pernicious Anemia |
|
|
What would comprise a positive Schilling Test?
|
Both free B12 and B12/IF in the urine
|
|
|
Pernicious Anemia:
-__1__ anemia due to __2__ deficiency resulting from lack of production of __3__ by the __4__ cells of the gastric mucosa -Accompanied by __5__, which is often seen first |
1. Megaloblastic
2. B12 (need it to make THF) 3. Intrinsic Factor 4. Parietal Cells 5. Achlorhydria (no HCl) = gastric infections |
|
|
What group of people is Pernicious Anemia generally observed?
|
Older men and women of Northern European decent (Scandinavians)
|
|
|
How much time may it take between the loss of Intrinsic Factor and the development of Megaloblastic Anemia?
Why? |
5 years
Thats how long it takes to deplete the stores of B12 from the Liver |
|
|
Treatment with __1__ should not be delayed after gastrectomy, and should be continued for __2__
|
1. parenteral B12
2. life |
|
|
List 3 things that could cause Bone Marrow failure, causing decreased RBC production and anemia
|
1. Myelofibrosis and Multiple Myeloma
2. Myelosuppressive Chemotherapy -Antitumor agents -drugs used to treat AIDS -Immunosuppressive agents 3. Deficiency of hematopoietic growth factors -chronic renal failure -> EPO deficiency |
|
|
This is a glycoprotein that stimulates red cell production and is derived from genetically modified cells of Chinese Hamster Ovary
|
Epoetin Alpha (Erythropoietin)
|
|
|
Under what 2 conditions is Epoetin alpha used?
|
1. Anemia patients with Chronic Renal Failure (kidney produces EPO)
2. Cancer patients receiving chemotherapy |
|
|
Recombinant granulocytic-macrophage colony stimulating factor (GM-CSF)
|
Sargramostim
|
|
|
When is Sargramostim used?
|
1. promotes myeloid recovery in patients with non-Hodgkin's Lymphoma, Acute Lymphoblastic Leukemia, and Hodgkin's disease who are undergoing Bone Marrow transplantation
2. promotes Myeloid Recovery after standard-dose chemotherapy 3. treats drug-induced bone marrow toxicity or neutropenia associated with AIDS |
|
|
Recombinant G-CSF used to prevent and treat chemotherapy-related febrile neutropenia, for promotion of myeloid recovery in patients undergoing bone marrow transplantation
|
Filgrastim
**Granulocytes = Neutrophils, Eosinophils, Basophils |
|
|
Promotes megakaryopoiesis and therefore platelet production
|
Oprelvekin (IL-11)
** vs. Anagrelide which inhibits Megakaryocyte development |
|
|
NSAID that is only analgesic and antipyretic, but not anti-inflammatory
|
Acetaminophen
|
|
|
NSAIDs:
-effects are due to inhibition of __1__ synthesis from __2__ |
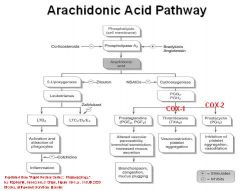
1. Eicosanoid
2. Arachidonic acid |
|
|
What 4 things are Prostaglandins involved in?
|
1. Pain (analgesic)
2. Fever (antipyretic) 3. Inflammation 4. Platelet aggregation |
|
|
Draw the Arachidonic Acid pathway
|
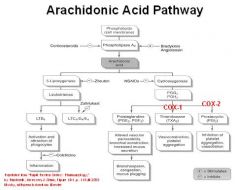
-
|
|
|
Aspirin and most other NSAIDs are weak bases or acids?
|
Weak Acids
|
|
|
Aspirin and other NSAIDs inhibit __1__ and __2__ biosynthesis but generally not __3__ synthesis
|
1. Prostaglandin
2. Thromboxane 3. Leukotriene |
|
|
What is the target of Aspirin?
|
Cyclooxygenase
|
|
|
Where is Cox-1 found and what does Aspirin inhibit the production of?
|
Most cells and Platelets --/ TXA2 production = vasodilation and anti-platelet aggregation
|
|
|
Where is COX-2 present and what does Aspirin inhibit the production of?
|
Inflammation cells and Endothelial Cells --/ PGI2 production
|
|
|
What are the new NSAIDs that selectively inhibit COX-2? (3)
|
1. Celecoxib (Celebrex)
2. Rofecoxib (Vioxx) 3. Valdecoxib ***all end in -ecoxib |
|
|
What is unique about Aspirin's mechanism of action
|
IRREVERSIBLY inhibits COX by Acetylating the enzyme
|
|
|
How is Aspirin different from Salicylic acid?
|
it is acetylated
|
|
|
Low doses of NSAIDs are given for __1__ and __2__ effects
High doses of NSAIDs are given for __3__ effects |
1. Analgesia (pain)
2. Antipyresis (fever) 3. Anti-inflammatory |
|
|
Most NSAIDs inhibit COX, but what 2 inhibit both COX and Lipoxygenase?
|
Ketoprofen
Indomethacin |
|
|
Why does Acetaminophen not inhibit Inflammation?
|
Inhibition of COX by Acetaminophen is blocked by Peroxides, which are formed during inflammation
|
|
|
What is the 1/2 life of Aspirin? Why?
|
15 min -> rapidly metabolized to Salicylic Acid = reversible inhibitor of COX
|
|
|
Salicylic acid is extensively conjugated with these 2 things
|
1. Glucuronic Acid
2. Glycine |
|
|
Explain the "dose-dependent pharmacokinetics" of Aspirin
|
When conjugation pathway become saturated, small increases in the dose of aspirin can produce large increases in plasma Salicylate levels
-low dose = first-order kinetics = Analgesic, Antipyretic -High dose = zero-order kinetics = Anti-inflammatory |
|
|
What dose of Aspirin gives Zero-order kinetics?
What is the Half-life? |
> 4000 mg = anti-inflammatory
> 12 hours |
|
|
What dose of Aspirin gives first-order kinetics?
What is the Half-life? |
600 mg = analgesic, antipyretic
3-5 hours |
|
|
How is Aspirin excreted?
|
In urine as Salicylic acid, Salicyluric acid, glucuronic acid conjugates
|
|
|
Aspirin Anti-inflammatory effects:
-due to inhibition of __1__ -__2__ synthesis is inhibited -Inhibits __3__ and __4__ migration to the site of inflammation |
1. COX-2
2. Prostaglandin 3. macrophage 4. lymphocyte |
|
|
Antipyretic effects of Aspirin:
-lowers body temp when __1__ is present -blocks production of __2__ in the CNS to "reset" temp control at the hypothalamus facilitating heat dissipation by __3__ |
1. fever
2. prostaglandins 3. vasodilation |
|
|
Antiplatelet effects of Aspirin:
-aspirin prolongs __1__ -Antithrombotic effect due to inhibition of __2__ synthesis via irreversible inhibition of platelet __3__ |
1. bleeding time
2. Thromboxane 3. COX-1 |
|
|
Effects of Aspirin on Uric Acid:
-Large doses of aspirin are __1__ -Lower doses __2__ uric acid levels |
1. uricosuric = decrease uric acid levels
2. increase **Large doses inhibit both secretion and reabsorption in the Kidney **Low doses inhibit only Secretion |
|
|
Why is Aspirin contraindicated in Gout?
|
b/c low doses increase uric acid levels
|
|
|
What is the main adverse effect of Aspirin when using usual dosages?
|
Gastric upset
|
|
|
What can minimize the gastric discomfort when taking aspirin?
|
taking with food or milk
|
|
|
Why does Aspirin cause GI effects?
|
Aspirin inhibits synthesis of prostaglandins, which are required for normal mucosal cell proliferation
|
|
|
Patients taking of any NSAID for prolonged periods may be given a PGE1 analog __1__, or proton pump inhibitors =__2__, to prevent peptic ulcers
|
1. Misoprostol
2. Omeprazole |
|
|
Why can Aspirin cause Renal Toxicity?
|
inhibits PGE2 and PGI2, which normally increase renal blood flow
When they are deficient = decreased renal blood flow -> HTN |
|
|
Large doses of Aspirin should be administered with caution in these types of patients? Why?
|
Diabetics
Can have an effect on Glucose tolerance |
|
|
Under what 3 conditions is Aspirin contraindicated?
|
1. Hemophiliacs = b/c aspirin has an antiplatelet effect
2. Patients with Aspirin-induced Nasal polyps or with allergic rxns (urticaria) 3. Children with viral infections due to associated risk of Reye's syndrome |
|
|
If you give Aspirin to a patient with Aspirin-induced Nasal Polyps or with Allergic rxns, what are they at risk of developing?
|
Bronchoconstriction
Anaphylaxis |
|
|
Aspirin overdose toxicity is a common cause of poisoning in ________
|
young children
|
|
|
What are the symptoms of Aspirin Overdose Toxicity?
|
1. Salicylism (tinnitus, vertigo, deafness)
2. Hyperthermia (aspirin uncouples oxidative phosphorylation) 3. Hyperpnea = rapid breathing 4. initially, respiratory alkalosis 5. Later, respirator and metabolic acidosis |
|
|
What is the treatment for Aspirin Overdose Toxicity?
|
1. if seen early, gastric lavage
2. maintain their temp (cool them down) 3. IV fluids 4. Bicarb + Potassium 5. Hemodialysis or Hemoperfusion, in severe cases |
|
|
What are the 3 uses of Aspirin?
|
1. Antipyretic = fever reducer
2. Acute Rheumatic Fever 3. Mild pain associated with inflammation -headache -myalgia = muscle pain -arthritis -Dysmenorrhea = painful period |
|
|
What 4 things can Aspirin be prophylatically be used for?
|
1. Platelet hyperaggregation
2. Coronary Artery disease 3. MI 4. Postoperative deep-vein thrombosis ***All Prophylaxes are for Anti-platelet effects |
|
|
What 5 drugs or group of drugs does Aspirin displace from Albumin and cause increased free concentration?
|
1. Oral Hypoglycemic drugs
2. Other NSAIDs 3. Methotrexate -> important b/c 50% is protein bound w/o aspirin and is intrinsically toxic 4. Phenytoin 5. ORAL ANTICOAGULANTS (WARFARIN) -> both anti-coagulant/anti-platelet = potential for bleeding *all exacerbated more with anti-inflammatory doses |
|
|
Other Nonselective NSAIDs:
-Antipyretic, analgesic, and anti-inflammatory effects similar to __1__ -All agents alter __2__ fxn and prolong __3__ -Treats mild to moderate pain, fever, and Rheumatoid Arthritis when patients cannot __4__ -Major differences are __5__ of action and potency |
1. Aspirin
2. platelet 3. bleeding time 4. tolerate aspirin 5. duration |
|
|
List other 6 NSAID drugs
|
NSAID "IS PAIN"
1. Ibuprofen 2. Sulindac 3. Piroxicam 4. Aspirin 5. Indomethacin 6. Naproxen |
|
|
Half-life and RA dosage:
1. Ibuprofen 2. Naproxen 3. Piroxicam |
1. 2 hours / 4 X 600mg
2. 14 hours / 2 x 375 mg 3. 45 hours / 1 x 20 mg |
|
|
What are 3 newer NSAIDs that are selective COX-2 inhibitors?
|
1. Celecoxib
2. Rofecoxib 3. Valdecocoxib **-ecoxib |
|
|
What is the major difference between Aspirin and the other NSAIDs?
|
Aspirin binds IRREVERSIBLY
the others bind reversibly |
|
|
These 2 NSAIDs were removed from the market b/c of increased CARDIAC events
What were the events likely due to? |
1. Rofecoxib & Valdecoxib
2. inhibition of PGI2 formation |
|
|
What NSAIDs inhibit both Cyclooxygenase and Lipoxygenase?
|
Ketoprofen
Diclofenac Indomethacin |
|
|
All NSAIDs except __1__ have a Black Box Warning of increased __2__ risks; likely due to inhibition of __3__
|
1. Aspirin
2. Cardiovascular 3. Renal Prostaglandins |
|
|
-One of the few NSAIDs that can be given parentally (IV, IM, PO) for moderate pain.
-Systemic use should not be more than 5 days. -Eye preparation is available for ocular pain. |
Ketorolac
*use dramatically cuts down the amount of narcotics needed post-surgically |
|
|
Indomethacin:
-not suggested as a general use __1__, but particularly effective for __2__ -Produces numerous __3__ effects and several severe __4__ reactions |
1. Analgesic
2. pain at night 3. GI 4. Hematopoietic |
|
|
What 4 things can Indomethacin be used to treat?
|
1. Acute GOUT
2. Ankylosing spondylitis 3. Osteoarthritis of the hip 4. PDA closure in premature infants |
|
|
What are some side effects of Indomethacin?
|
1. Headache
2. Indigestion 3. CNS - vertigo - Dizziness - confusion |
|
|
With the exception of newborns with __1__, Indomethacin is not given to children. Nor is it given to __2__
|
1. Patent Ductus Arteriosus
2. Pregnant women = don't want the PDA to close in utero |
|
|
Celecoxib:
-selective antagonist of __1__ -does not inhibit __2__ and does not cause __3__ |
1. COX-2 (found in Inflammatory cells and mediates inflammation and pain)
2. platelet function 3. gastropathy |
|
|
When is Celecoxib contraindicated?
|
patients with known Celecoxib hypersensitivity or SULFONAMIDE hypersensitivity
- Celecoxib contains a Sulfonamide side chain |
|
|
What is Celecoxib's approved uses? (2)
What things is it being studied to treat? (4) |
Approved:
1. Rheumatoid Arthritis 2. Osteoarthritis Studies: 1. sporadic adenomatous polyps of the colon 2. Barrett's esophagus 3. Actinic keratosis 4. superficial bladder cancer |
|
|
Trade name for Rofecoxib
|
Vioxx
|
|
|
Why was Rofecoxib removed from the market?
|
Selective COX-2 inhibitor
-inhibits PGI2 formation but causes a higher amount of TXA2 production from COX-1 pathway -TXA2 = vasoconstriction and platelet aggregation |
|
|
What does Rofecoxib use have an increased risk of causing?
|
Cardiovascular events with prolonged use and high doses
|
|
|
Before being removed from the market,what was Rofecoxib indicated for?
|
1. to relieve the signs and symptoms of Osteoarthritis
2. treatment of Dysmenorrhea or acute pain |
|
|
Rofecoxib:
-compared to __1__, it lacks a __2__ and thus is not contraindicated in patients allergic to __3__ |
1. Celecoxib
2. Sulfonamide 3. Sulfonamides |
|
|
What were 2 advantages of Rofecoxib?
|
1. no sulfonamide hypersensitivities
2. not primarily dependent on CYP450 enzymes = no drug-drug interactions |
|
|
-Selective COX-2 inhibitor that was withdrawn from the market
-was contraindicated in patients with Sulfonamide hypersensitivity |
Valdecoxib
|
|
|
What was Valdecoxib indicated for before being taken off the market? (3)
|
1. Dysmenorrhea
2. Osteoarthritis 3. Rheumatoid arthritis *Dysmenorrhea is caused by excessive prostaglandin synthesis |
|
|
Why was Valdecoxib taken off the market?
|
Shifts the TXA2/PGI2 balance to an unfavorable ratio = increased risk for CV events
|

