![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
98 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Define Tolerance
|
response diminishes with time
|
|
|
|
What is Pharmacokinetic tolerance due to?
|
induction of drug metabolizing enzymes
|
|
|
|
Alternate names for Pharmacokinetic tolerance
|
Metabolic tolerance
Drug disposition tolerance |
|
|
|
Give an example of Pharmacokinetic Tolerance
|
Warfarin, an oral anticoagulant, dose needs to be increased in a patient taking barbiturates or Phenytoin
|
|
|
|
Explain Pharmacodynamic tolerance
|
-develops at the cellular level
-is due to changes in receptor numbers or function |
|
|
|
List the 2 mechanisms of Pharmacodynamic tolerance
|
Desensitization
Down-regulation |
|
|
|
Explain Desensitization
|
process occurs rapidly when continuous exposure to an agonist results in 1) conversion of a channel to an altered state that remains closed or 2) when a receptor-coupling element is phosphorylated to an inactive form
|
|
|
|
What cause receptor down-regulation
|
Agonists
|
|
|
|
Explain Down-regulation
|
process of ligand-induced endocytosis and degradation of receptor, caused by agonists when administered at high doses for a prolonged period
|
|
|
|
Give 3 examples of Pharmacodynamic Tolerance
|
1. continuous exposure to beta-adrenergic agonists,such as occurs in treatment of asthma with ALBUTEROL, results in a decreased responsiveness to the drug
2. tolerance to the analgesic effects of MORPHINE upon continued use 3. decreased sedation from continuous treatment with a Benzodiazepine such as DIAZEPAM |
|
|
|
Explain Physiological Antagonism
|
when 2 agents have opposing physiological effects
|
|
|
|
Give an example of Physiological antagonism
|
Histamine causes Vasodilation while NE causes Vasoconstriction -> when given together they counteract eachother
|
|
|
|
Explain Competitive Antagonism
|
when a receptor antagonist is administered with an agonist
|
|
|
|
Competitive Antagonism: what does Naloxone block the effect of?
|
Morphine
|
|
|
|
Competitive Antagonism: what does Atropine block the effect of?
|
ACh at muscarinic receptor
|
|
|
|
Competitive Antagonism: what does Propranolol block the effect of?
|
Isoproterenol at beta-adrenergic receptors
|
|
|
|
Describe how Supersensitivity or Hyperactivity occurs
|
due to an increase in the number of receptors
-during absence of ligand -prolonged presence of antagonist |
|
|
|
Give an example of Chemically induced supersensitivity
|
after prolonged treatment with Beta-blockers some individuals become supersensitive to endogenous release of Catecholamines (Epi, NE, Dopa)
|
|
|
|
Explain Denervation induced Supersensitivity
|
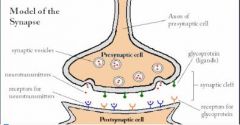
occurs at POST-synaptic receptors when Pre-synaptic nerve is surgically destroyed or lesioned
|
|
|
|
What causes receptor up-regulation?
|
Antagonists
|
|
|
|
Pharmacogenetic cause of increased activity of drugs
|
deficiency in degrading enzymes
|
|
|
|
Patients with abnormal serum cholinesterase have increased sensitivity to what drug?
|
Succinylcholine = muscle relaxant
|
|
|
|
Individuals with Glucose-6-phosphate Dehydrogenase deficiency develop Acute Hemolytic Anemia after given what drug?
|
Primaquine
|
|
|
|
Explain how competition for binding sites can result in increased activity of a drug
|
drugs may displace one another from plasma albumin binding sites, enhancing response to one or both agents
|
|
|
|
T or F: If a drug is displaced from a plasma protein-binding site, response is INTENSIFIED and duration of action is PROLONGED
|
False: duration is shortened
|
|
|
|
Explain how Physiological Synergism can increase activitiy of drugs
|
when 2 drugs produce the same or similar effects through different receptors or mechanisms
|
|
|
|
Explain an additive effect
|
when two drugs are given and their effect is like adding their individual effects together
5+5 = 10 |
|
|
|
Classic Example of 2 drugs that act synergistically
|
Diazepam + Ethanol produce severe, prolonged CNS depression
|
|
|
|
Explain Synergistic effect
|
When two drugs are added together and their effects together are greater than when adding their effects alone
5 + 5 = 15 |
|
|
|
Explain Potentiation
|
one drug might not have an effect alone but in combo with other drugs it
has an effect 0+5 = 20 |
|
|
|
Definition: repeated administration produces altered or adaptive physiological state and physiological disturbances (withdrawal, abstinence syndrome) occur if drug is not present
|
Physical Dependence
|
|
|
|
Drugs that result in dependence (4)
|
1. Alcohol
2. Barbiturates 3. Narcotic analgesics 4. Nicotine |
|
|
|
Definition: Compulsive drug-seeking behavior; individual uses drug repetitively for personal satisfaction
|
Psychological dependence
|
|
|
|
Definition: cluster of symptoms indicating that the individual continues substance use despite significant substance-related problems
|
Substance dependence (addiction)
|
|
|
|
Overextension of Pharmacological response: Atropine induces _______
|
Dry mouth
|
|
|
|
Overextension of Pharmacological response: Propranolol induced _______
|
Heart Block
|
|
|
|
Overextension of Pharmacological response: Diazepam-induced _______
|
Drowsiness
|
|
|
|
Organ-directed toxicities: Aspirin-induced _______
|
GI toxicity
|
|
|
|
Organ-directed toxicities: Aminoglycoside-induced ______
|
Renal toxicity
|
|
|
|
Organ-directed toxicities: Acetaminophen-induced ______
|
Hepatotoxicity
|
|
|
|
Organ-directed toxicities: Doxorubicin-induced ______
|
Cardiac toxicity
|
|
|
|
Direct fetal toxic effects: Sulfonamide-induced ______
|
Kernicterus = damage to the brain centers of infants caused by jaundice
|
|
|
|
Direct fetal toxic effects: Chloramphenicol-induced ________
|
Gray baby syndrome
|
|
|
|
Direct fetal toxic effects: Tetracycline-induced _______
|
-teeth discoloration
-Retardation of bone growth |
|
|
|
When are Teratogenic effects most pronounced?
|
during organogenesis: day 20 - end of 1st trimester
|
|
|
|
List 8 human teratogens
|
1. Thalidomide
2. Antifolates 3. Phenytoin 4. Warfarin 5. Isotretinoin 6. Lithium 7. Valproic acid 8. Fetal alcohol syndrome |
VALerie is an ANTIFOLATE from THAILAND. Her country is at WAR with the PHENYTOIN's. They give their babies LITHIUM AND ISOTRETINOIN which yields FETAL ALCOHOL SYNDROME
|
|
|
Definition: Abnormal response resulting from previous sensitizing exposure activating immunologic mechanism
|
Drug allergies (hypersensitivity)
|
|
|
|
T or F: in drug allergies, altered reaction occurs only in a fraction of the population
|
T
|
|
|
|
T or F: In drug allergies the dose-response is unusual: a large amount of an otherwise safe drug elicits severe reaction
|
False: minute amounts elicit a severe reaction
|
|
|
|
T or F: In drug allergies the manifestations of reaction are similar to the pharmacological and toxicological effects of the drug
|
False: they are different
|
|
|
|
T or F: Drug allergies
-Primary sensitization period occurs before the individual experiences the response |
True
|
|
|
|
T or F: Drug allergies
-Most drug by themself are immunogenic |
False: Being small molecules, most drugs by themselves are not immunogenic; they bind covalently to self-macromolecule or alter structure of self-macromolecule to become immunogenic
|
|
|

Fill in the table
|
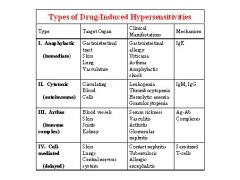
-
|
|
|
|
Explain the Type I Hypersensitivity reaction
|
-IgE molecules bind to blood basophils and tissue mast cells via Fc receptors for antibody
-When offending drug introduced into body, immediately binds to IgE bound to sensitized cells, resulting in release of mediators (histamine, leukotrienes, prostaglandins) -Mediators initiate skin and smooth muscle responses, cause tissue injury and provoke inflammatory response -Include anaphylaxis, urticaria and angioedema |
|
|
|
Explain Type II Hypersensitivity reactions
|
mediated by IgM or IgG binding to cells or tissues, resulting in the activation of complement and lysis of the cell
|
|
|
|
Explain Type III Hypersensitivity reactions
|
mediated by Immune complexes
|
|
|
|
What are symptoms of Immune-complex induced serum sickness?
|
-Urticarial skin eruptions
-Arthralgia -Lymphadenopathy -Fever |
|
|
|
What is the name of the severe form of immune vasculitis caused by drugs?
What drug can induce it? |
Stevens-Johnson syndrome
Sulfonamides |
|
|
|
Explain Type IV Hypersensitivity reaction
|
cell-mediated or delayed hypersensitivity, often occurs when drugs are applied topically
|
|
|
|
Type II reactions: Penicillin-induced _________
|
hemolytic anemia
|
|
|
|
Type II reactions: Methyldopa-induced __________
|
autoimmune hemolytic anemia
|
|
|
|
Type II reactions: Quinidine-induced _______
|
Thrombocytopenia
|
|
|
|
Type II reactions: Sulfonamide-induced ________
|
Granulocytopenia
|
|
|
|
5 drugs that can induce Type III Immune Vasculitis
|
Penicillin
Anticonvulsants Iodides Sulfonamides Thiouracils **PAIST** |
|
|
|
Definition: Abnormal response not immunologically mediated; often caused by genetic abnormalities in enzymes or receptors; referred to as pharmacogenetic disorders.
|
Drug Idiosyncrasies
|
|
|
|
Classical idiosyncracy: patients with abnormal serum Cholinesterase develop _____ when given normal doses of succinylcholine
|
Apnea = temporary suspension in breathing
|
|
|
|
"Slow" acetylators of Isoniazid are homozygous autosomal recessive for this gene's protein
|
N-acetyltransferase
|
|
|
|
What are "slow" acetylators of isoniazid more prone to?
|
isoniazid-induced Vitamin B6 deficiency which may produce:
-anemia -various neuropathies **Supplement with B6 when treating TB with Isoniazid |
|
|
|
Hemolytic anemia in patients with G-6-P Dehydrogenase can be induced by these 3 drugs
|
1. Primaquine
2. Sulfonamides 3. Nitrofurantoin |
|
|
|
_______-induced Porphyria occurs in individuals with abnormal ______ biosynthesis
|
Barbiturate
Heme |
|
|
|
Explain how Barbiturates can induce Porphyria
|
-the Barbiturate acid moeity mimics part of the Heme structure, occupying a portion of the heme site on the protein that regulates production of ALA synthetase
-Heme is a repressor, inhibiting production of ALA synthetase and reducing porphyrin production = without Heme present inhibiting the production of ALA synthetase, Porphyrin production will increase |
|
|
|
Definition: Application of the principles of pharmacokinetics, pharmacodynamics and adverse effects to the treatment of patients
|
Pharamcotherapeutics
|
|
|
|
The incidence of a placebo response is constant between what percents in clinical trials?
|
20 and 40%
|
|
|
|
Drug whose resin binds with other drugs and prevents their
|
Cholestyramine
|
|
|
|
Antacids have metals that chelate with these 2 drugs and prevent their absorption
|
Tetracyclines
Fluoroquinolones |
|
|
|
Group of drugs that decrease GI motility and slow absorption of many drugs
|
Anticholinergics (Atropine)
|
|
|
|
GI motility alteration may alter absorption of some of this group of drugs
|
Weak organic acids
|
|
|
|
3 groups of plasma protein binding drugs that can displace drugs from albumin
|
1. Oral hypoglycemic agents (tolbutamide)
2. Oral anticoagulants (warfarin) 3. Antimetabolites (methotrexate) |
|
|
|
Digoxin can be displaced from its tissue binding sites by this drug
|

Quinidine
|
|
|
|
Alkalinization of urine (Sodium Bicarb) enhances excretion of ________
|
Weak organic acids
|
|
|
|
Acidification (ammonium chloride) of urine enhances the excretion of _______
|
weak organic bases
|
|
|
|
What drug blocks the tubular excretion of penicillin?
|
Probenicid
|
|
|
|
Administration of these two nephrotoxic drugs can produce kidney damage even when dose of either agent alone may have been insufficient to produce toxicity
|
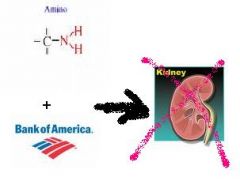
Aminoglycoside
Vancomycin |
|
|
|
What 2 properties of drugs are regulated by the FDA?
|
Safety
Efficacy |
|
|
|
What is filed with the FDA once a potential drug is judged ready to be initiated for Human studies
|
Investigational New Drug (IND) exemption
|
|
|
|
What is the goal in Phase 1 of Human Testing?
|
to find the maximum tolerated dose = "Is it safe?"
|
|
|
|
What is always the first dose in Phase 1 human testing?
|
Placebo
|
|
|
|
T or F: testing is never double-blind in Phase 1 testing
|
True
|
|
|
|
What is the definition of Phase 2 human testing?
|
first attempt to determine clinical effectiveness of test agent = "Does it work?"
|
|
|
|
T or F: Phase 2 tests may be single-blind or double-blind and involve hundreds of patients
|
True
|
|
|
|
What is the definition of Phase 3 human testing?
|
extensive testing of a drug's efficacy and toxicity = "How well does it work, and what are the common side effects?"
|
|
|
|
Phase 1 and 2 studies are usually conducted by __1__, but Phase 3 may include __2__
|
1. Clinical scientists
2. physicians in private practice |
|
|
|
After completion of Phase 3 testing, company files _________with the FDA
|
New Drug Application
|
|
|
|
Fewer than how many subjects are usually tested before Phase 4?
|
10,000
|
|
|
|
What is Phase 4 human testing?
|
post-marketing surveillance = when adverse effects and toxicity become most evident
|
|
|
|
During which Phases is informed consent required?
|
Phase 1-3
|
|
|
|
Informed consent must be in writing for which Phases?
|
1 and 2
|
|
|
|
Who protects the interest of the subjects in Phase testing?
|
Peer review (Committee on Human Experimentation)
|
|

