![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
100 Cards in this Set
- Front
- Back
|
Anti-HIV Fusion Inhibitor
|
Enfuvirtide (T-20)
|
|
|
What is Enfuvirtide's mechanism of action?
|
binds gp41 of HIV envelope, blocking fusion of viral and host cell membranes
|
|
|
Enfuvirtide:
1. type of compound 2. administration 3. half-life 4. blocks infection of ____ |
1. Synthetic peptide (36 aa's)
2. Subcutaneous injection 3. 4 hours 4. new cells |
|
|
Alternate name for Zidovudine
|
Azidothymidine (AZT)
|
|
|
NRTI Thymidine analog
|
Zidovudine (AZT)
Stavudine (d4T) |
|
|
Zidovudine pharmacokinetics:
1. Distribution 2. Downside |
1. Widely distributed, CNS 60% of serum level
2. rapidly metabolized and excreted -half life = 1-3 hours - urine, 75% as glucuronide - Competitive inhibitor so need to maintain levels to have therapeutic effect |
|
|
What is Zidovudine an analog of?
|
Deoxythymidine
|
|
|
What is the clinical use of Zidovudine?
|
-Temporarily reduces morbidity and mortality from HIV
-used in 3 drug cocktail (HAART) -reduces mother-to-newborn transmission -Prophylaxis **inhibits infection of new cells |
|
|
How is resistance to Zidovudine to attained?
|
mutations in RT
|
|
|
NRTI with these ADR's that are common and may be very severe:
1. Bone marrow depression 2. CNS = headaches, agitation, insomnia |
Zidovudine
|
|
|
How can toxicity to Zidovudine be enhanced?
|
by drugs which compete for glucuronidation = that is how the drug is cleared
|
|
|
What is the abbreviation of Didanosine?
|
dideoxyinosine (ddI)
|
|
|
How is Didanosine administered?
|
Oral, but acid labile
|
|
|
What is the mechanism of Didanosine?
|
RT inhibitor
- incorporated into viral DNA to cause chain termination |
|
|
T or F: Didanosine may not be cross-resistant with AZT
|
True = viruses resistant to AZT may not be resistant to ddI
|
|
|
Anti-HIV that may cause:
1) Dose-dependent Pancreatitis 2) Peripheral neuropathy |
Didanosine
|
|
|
What is the abbreviation for Stavudine?
|
d4T = analog of thymidine
|
|
|
Stavudine is similar to ddI (Didanosine), but is different in what way?
|
Higher bioavailability
|
|
|
NRTI that readily crosses the BBB
|
Stavudine
|
|
|
NRTI with these ADR's:
1. Peripheral neuropathy 2. Lactic acidosis |
Stavudine
|
|
|
What is Zalcitabine?
|
Nucleoside RT Inhibitor
ddC |
|
|
NRTI with these properties:
-actively transported into cells -resistant to hepatic metabolism -high bioavailability -dose-dependent Peripheral neuropathy -phenotypic resistance is rare |
Zalcitabine (ddC)
|
|
|
Abacavir is an analog of?
|
Guanosine
|
|
|
NRTI that is a Guanosine analog and resistance to it requires 2-3 concomitant mutations (develops slowly)
|
Abacavir
|
|
|
What does resistance to Abacavir require?
|
2-3 concomitant mutations = develops slowly
|
|
|
NRTI with these ADR's:
1. Skin rash in 50% 2. Hypersensitivity in 5% (can be fatal) |
Abacavir
|
|
|
1st generation oral Non-nucleoside RT Inhibitor
|
Nevirapine
|
|
|
How do NNRTI's work?
|
Non-competitive (allosteric) inhibitors of Reverse Transcriptase
- binds near but not active site |
|
|
What is the downfall of Nevirapine? What is done to prevent it?
|
RAPID emergence of resistance
Only approved for combination therapy with NRTI's |
|
|
What is the exception to giving Nevirapine in combination therapy?
|
Single doses are given to mother and neonate
|
|
|
T or F: Nevirapine cannot be active against AZT-resistant HIV
WHY? |
False: it can be active against AZT-resistant HIV b/c they bind different sites on RT
|
|
|
Protease Inhibitor that has these ADR's:
1. Skin rash - 17% 2. Hepatotoxicity - 4% |
Nevirapine
|
|
|
Anti-HIV that is both a substrate for and inducer of CYP 3A4
|
Saquinavir
|
|
|
What is the ending of all Protease Inhibitors?
|
-navir
*Never (navir) tease a pro (protease inhibitors) |
|
|
Describe how HIV Protease Inhibitors work
|
-HIV proteins are translated as a polyprotein
- Must be cleaved to yield active individual proteins -PI's inhibit the action of the Protease = prevent viral assembly and release of infectious particles |
|
|
What type of protease is the HIV protease?
|
Aspartyl protease (aspartic acid is in active site)
|
|
|
What are the adverse effects of Protease Inhibitors
|
1. Altered body fat distribution
- girdle of fat around hips - extensive deposits in face -"buffalo hump" 2. Hyperlipidemia 3. Insulin resistance **PUDGY Protease Inhibitors |
|
|
1st HIV Protease Inhibitor on the market
|
Saquinavir
|
|
|
Protease Inhibitor that is a good substrate for "first-pass" metabolism b/c it is a substrate for and inducer of CYP 3A4 = 95% metabolized before reaching systemic circulation
|
Saquinavir
|
|
|
How does resistance to Saquinavir develop?
|
Via mutations in HIV Protease
*but SLOWLY!!! |
|
|
What do you use HIV Protease Inhibitors in combination with?
|
NRTI's
|
|
|
HIV Protease Inhibitor with the highest CNS levels
|
Indinavir
|
|
|
HIV Protease Inhibitor that may cause:
-Hyperbilirubinemia -Nephrolithiasis = renal calculi |
Indinavir
|
|
|
HIV Protease Inhibitor that currently has the highest levels in the CNS
|
Indinavir
|
|
|
Protease Inbititor metabolized by CYP 3A4 and CYP 2D6
*but not as an efficient substrate as other PI's for CYP's |
Ritonavir
|
|
|
Combination of HIV Protease Inhibitors where one blocks CYP 3A, allowing the other protection from 1st Pass metabolism
|
Lopinavir and Ritonavir (blocks CYP3A)
|
|
|
-ORAL HIV protease inhibitor, rapidly absorbed but extensively metabolized (CYP 3A4)
-Causes gastrointestinal effects: Nausea, vomiting, diarrhea, abdominal pain |
Lopinavir
|
|
|
What is Interfefon-alpha used to treat?
|
Hep B (management) and C (eradication with Ribavirin)
|
|
|
How is Interferon-alpha administered?
|
Subcutaneous or IM injection 3-7 times weekly
|
|
|
Describe the Pegylated form of INF-alpha?
|
provides a longer half-life (weekly administration vs. 3-7 / wk)
|
|
|
What is the most common side effect of IFN-alpha?
|
Flu-like syndrome
|
|
|
What is Lamivudine an analog of?
|
Cytosine
|
|
|
Antiviral with these 2 actions:
1. HIV NRTI 2. HBV DNA Polymerase Inhibitor |
Lamivudine
|
|
|
Lamivudine:
1. administration? 2. excretion? |
1. oral
2. unchanged in urine |
|
|
Lamivudine half-lives:
1. Serum? 2. Cellular triphosphate in HIV infected cells? 3. Cellular triphosphate in HBV-infected cells? |
1. 2.5 hrs
2. 10-15 hrs 3. 17-19 hrs |
|
|
Resistance to this antiviral is associated with Hepatitis flares and progression of Liver damage
|
Lamivudine
*NRTI & HBV DNA Pol Inhibitor |
|
|
Pegylated IFN-alpha plus ______ is standard of care for eradication of HCV
|
Ribavirin
|
|
|
3 drugs used in Anti-Influenza chemotherapy
|
1. Rimantidine
2. Oseltamivir (Tamiflu) 3. Zanamavir (Relenza) |
|
|
What is Rimantadine's mechanism of action?
|
blocks Influenza's uncoating by blocking M2 proton channel
***"A man to dine" takes off his COAT |
|
|
When is Rimantadine supposed to be given?
|
in first 48 hours after contact
|
|
|
What are the adverse effects of Rimantadine?
|
GI intolerance
CNS effects **"A man to dine" has UPSET STOMACH and SLURRED SPEECH |
|
|
What is Rimantadine clinically used for?
|
INfluenza A
|
|
|
What are the 2 Influenza Neuraminidase Inhibitors?
|
Oseltamivir (Tamiflu)
Zanamivir (Relenza) **AMIVIR's |
|
|
Describe the mechanism of action Influenza Neuraminidase Inhibitors
|
Prevent viral release from infected cells = prevents infection of additional cells
*causes viral clumping at surface |
|
|
First oral agent effective against all common Influenza strains
|
Oseltamivir (Tamiflu)
|
|
|
-Pro-drug; activated in the gut and liver = can only give orally
-Well tolerated, some transient nausea and vomiting -Anti-influenza drug |
Oseltamivir (Tamiflu)
|
|
|
Zanamavir:
1. Administration? 2. Active against? 3. Excretion? 4. When effective? 5. Adverse effect |
1. Oral inhalation
2. Influenza A and B 3. urine, unchanged 4. early in infection (>2 days) 5. Bronchospasm in asthmatics |
|
|
List the 5 general characteristics of tumor cells
|
1. Excessive/inappropriate growth
2. diminished apoptosis 3. loss of differentiation 4. Invasive 5. Metastatic |
|
|
What is Multi-modality therapy?
|
Chemotherapy + Surgery
|
|
|
# of tumor cells that is a fatal burden?
|
10^12
|
|
|
# of tumor cells that elicit unmistakable symptoms?
|
10^11
|
|
|
# of tumor cells correlating with the Subclinical range?
|
10^9 and below
|
|
|
Cure requires "total Kill":
-must kill all cells with __1__ - __2__ detection/eradication of tumor cells is very poor |
1. Proliferative potential
2. Immune |
|
|
Chemotherapy following surgery or radiation
|
Adjuvant
|
|
|
Chemotherapy before surgery or radiation
|
Neoadjuvant
|
|
|
Variable Success Rates in Cancer Treatment:
-About 50% of cancers can be cured using __1__ therapy -About 17% can be cured by __2__ alone -highly variable by __3__ |
1. Multi-modality
2. Chemotherapy 3. Tumor site/type |
|
|
List the groups of Anti-cancer drugs that are Cell-cycle specific
|
1. Antimetabolites = nucleoside analogs = S phase
2. Topoisomerase inhibitors 3. Microtubule poisons 4. Bleomycin |
|
|
What groups of anti-cancer drugs are effective during any stage of the cell cycle?
But what types of cells are they most effective against? |
1. Alkylating agents
2. Antitumor antibiotics Rapidly cycling cells |
|
|
What is the target for selective toxicity with Anti-cancer agents?
|
Tumor cells have a high proliferative potential
|
|
|
List the groups of cells/body sites that also have high proliferative potential
|
1. Bone marrow
2. Epithelial cells 3. Immune system 4. Hair follicles |
|
|
What do most effective anticancer drugs activate?
|
Apoptosis
|
|
|
Apoptosis does not occur in the absence of functional _____
|
p53
*tumors that have lost this will be far more resistant to the effects of cytotoxic anti-cancer drugs |
|
|
What is the most prominent acute adverse effect of anti-tumor drugs?
|
Nausea and Vomiting
|
|
|
How can nausea and vomiting be treated when giving anti-tumor drugs?
|
Treat with Antiemetics
1. 5-HT3 antagonists = Ondansetron 2. Phenothiazines *30% unaffected by antiemetics |
|
|
What are the two "-penia's" that usually occur first as a delayed effect of Anti-tumor drugs? What do they put you at risk for?
|
1. Leukopenia (neutropenia) = risk of infection
2. Thrombocytopenia = risk of bleeding |
|
|
How do you treat the Myelosuppression effects of anti-tumor drugs? (3)
|
1. Leukopenia with GM-CSF
2. Thrombocytopenia with Platelet transfusions 3. Anemia with Erythropoietin |
|
|
Why are there GI effects with anti-tumor drugs?
|
b/c we have an epithelial cell population that has to be replenished every day
|
|
|
What is "Hand and Foot Syndrome" associated with some Anti-tumor drugs
|
Edema, pigmentation, and neural changes in the hands and feet
|
|
|
Describe Secondary Malignancy associated with Anti-cancer drugs
|
- many anti-cancer drugs act by modification of nuclear DNA, leading to altered structure and fxn
-therapy which helped cure the primary malignancy may cause a secondary malignancy years later -usually a lag period of 10-20 years |
|
|
When is Secondary Malignancy less of a concern?
|
In a person who is older
*a younger person has whole life to develop secondary malignancy |
|
|
Since anti-cancer chemotherapy selects for resistance, what is the approach used?
|
Combination chemotherapy
|
|
|
Describe Primary Resistance of tumor cells
|
tumor cells are initially not sensitive to a given drug therapy
*can bypass this by IN VITRO sensitivity testing |
|
|
Describe Secondary Resistance of tumor cells
|
tumors develop resistance during therapy
-changes in: permeability, amplification/alteration of targets, enhanced repair |
|
|
What confers MULTI-DRUG RESISTANCE to Anti-cancer drugs?
|
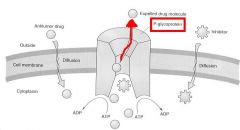
Active export by P-glycoprotein (MDR-1) = pumps out drugs
|
|
|
List the 7 major classes of Antineoplastic Drugs
|
1. Alkylating agents
2. Antimetabolites 3. Plant Alkaloids 4. Antitumor Antibiotics 5. Hormones and Hormone Modulators 6. Antibodies 7. Signal Transduction inhibitors |
|
|
Alkylating agents:
-__1__ bind to/modify biological molecules -__2__ is the key target -must be __3__--inherent, or generated by metabolism -__4__, but replicating cells are more sensitive |
1. Covalently
2. DNA 3. Reactive 4. Cell cycle non-specific |
|
|
Why are Alkylating agents more effective when cells are replicating?
|
b/c it decreases the chances that the adduct will be repaired
|
|
|
The differences in clinical uses of Akylating agents primarily reflects the ______ differences of each drug
|
pharmacokinetic
|
|
|
What is the mechanism of action of Alkylating agents?
|
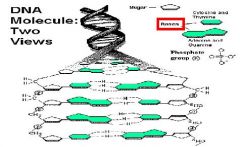
Covalently bind to BASES in DNA
|
|
|
Explain the "bifunctionality" of Alkylating agents
|
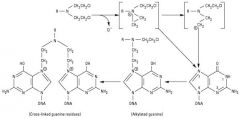
they latch on to a DNA base with one arm and then latch on to another piece of DNA with another part = blocks ability to read through that strand
|

