![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
The melting points vary because:
|
The oxides have different bondings and structures.
|
|
|
A graph of the melting points:
|
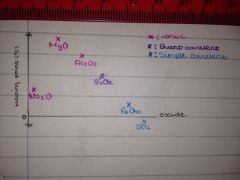
|
|
|
The ionic lattices are:
|
• Na2O
• MgO • Al2O3 |
|
|
The ionic oxides have high melting points because:
|
They have strong electrostatic forces of attraction between the oppositely charged ions.
|
|
|
Mg has a higher melting point than Na because:
|
Mg has a greater charge (+2) therefore it has a greater attraction with the –2 of the Oxygen.
|
|
|
Al2O3 has a lower melting point than expected:
|
The +3 charge on the Al polarises the anion creating a partial covalent bond which has a lower melting point than if it was purely ionic.
|
|
|
SiO2 has the highest melting point of the non-metals because:
|
It has a macromolecular structure which is made up of strong covalent bonds.
|
|
|
P4O10 & SO2 have the weakest melting points because:
|
They have simple molecular structures which are attracted weakly by intermolecular forces. (VDW & D-D)
|

