![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
Displayed formula |
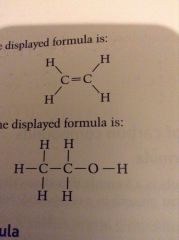
Shows all bonds |
|
|
Structural formula |

Shows the separate arrangement of molecules |
|
|
Naming numbers |
Meth one Eth Two Pro Three Tetr Four Pent. Five Hex. Six |
|
|
Prefixes. |
Methyl. Ethyl. Propyl. Butyl. Pentyl. Hexyl. |
|
|
Suffixes and functional groups |
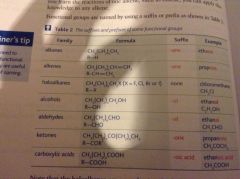
Back (Definition) |
|
|
Homologous series |
Is a family of organic compounds with the same functional groups but different chain length. |
|
|
Properties of a homologous series |
All differ by CH2 Length effects the chemical reactivity of the functional groups Length of the carbon chain effects physical properties. Melting and boiling points increase as intermolecular forces increase. Chain branching reduces boiling point as they're more packed together. |
|
|
Positional group isomerism |
The functional group is at different points on the chain |
|
|
Functional group isomerism |

Have different functional groups |
|
|
Chain isomerism |
The chain is arranged differently example butanol and 2-methylpropan-1-ol. |
|
|
Why is calcium oxide used to neutralise sodium dioxide , why is it on mesh |
It is basic increase surface area. |
|
|
Hydrocarbon
|
is a compound consisting of hydrogen and carbon only
|
|
|
Saturated:
|
Contain single carbon-carbon bonds only
|
|
|
Unsaturated :
|
Contains a C=C double bond
|
|
|
Petroleum fraction
|
mixture of hydrocarbons with a similar chain length and boiling point range
|
|
|
Molecular formula:
|
The formula which shows the actual number of each type of atom
|
|
|
Empirical formula:
|
shows the simplest whole number ratio of atoms of each element in the compound
|
|
|
General formula:
|
algebraic formula for a homologous series e.g. CnH2n
|
|
|
Displayed formula:
|
show all the covalent bonds present in a molecule
|
|
|
what is a homologous series |
Homologous series are families of organic compounds with the same functionalgroup and same general formula.•They show a gradual change in physical properties (e.g. boiling point).• Each member differs by CH2from the last.• same chemical properties.
|
|
|
Functional group
|
is an atom or group of atoms which when present in different moleculescauses them to have similar chemical properties
|
|
|
isomers structural isomers |
same molecular formula different structures (or structural formulae) Structural isomerism can arise from •Chain isomerism •Position isomerism •Functional group isomerism
|
|
|
Chain isomers |
Compounds with the same molecular formula but differentstructures of the carbon skeleton
|
|
|
position isomers:
|
Compounds with the same molecular formula but different structuresdue to different positions of the same functional group on the same carbon skeleton
|
|
|
functional group isomers |
Compounds with the same molecular formula but with atoms arranged to give different functional groups
Note: alkene and cyclo alkanes have the same general formula. Hexene and cyclohexane have the same molecular formula but have a different functional group |
|
|
Refining crude oil
Fractional Distillation: Petroleum fraction: |
Petroleum is a mixture consisting mainlyof alkane hydrocarbons
mixture of hydrocarbons witha similar chain length and boiling point range |
|
|
Refining crude oil Fractional Distillation:
|
Oil is pre-heated • then passed into column. • The fractions condense at different heights • The temperature of column decreases upwards • The separation depends on boiling point. • Boiling point depends on size of molecules. • The larger the molecule the larger the van der waals forces • Similar molecules (size, bp, mass) condense together • Small molecules condense at the top at lower temperatures • and big molecules condense at the bottom at higher temperatures
|
|
|
what is the physical process |
This is a physical processinvolving the splitting ofweak van der waals forcesbetween molecules
|
|
|
Vacuum distillation unit
|
Heavy residues from the fractionating column are distilledagain under a vacuum.• Lowering the pressure over a liquid will lower its boilingpoint
Vacuum distillation allowsheavier fractions to be furtherseparated without hightemperatures which couldbreak them down |
|
|
cracking |
conversion of large hydrocarbons to smaller hydrocarbon molecules by breakage of C-C bonds
High Mr alkanes smaller Mr alkanes+ alkenes + (hydrogen) |
|
|
Economic reasons for cracking
|
The petroleum fractions with shorter C chains (e.g. petrol and naphtha) are in more demand than larger fractions. • To make use of excess larger hydrocarbons and to supply demand for shorter ones, longer hydrocarbons are cracked. • The products of cracking are more valuable than the starting materials (e.g. ethene used to make poly(ethene), branched alkanes for motor fuels, etc.)
|
|
|
what does cracking include |
This is a chemical processinvolving the splitting ofstrong covalent bonds sorequires high temperatures
|
|
|
thermal cracking |
Conditions: High Pressure (7000 kPa) High Temperature (400°C to 900°C)
produces mostly alkenes e.g. ethene used for making polymers and ethanol sometimes produces hydrogen used in the Haber Process and in margarine manufacture C8H18 C6H14 + C2H4C12H26 C10H22 + C2H4 Bonds can be broken anywhere in the moleculeby C-C bond fission and C-H bond fission. |
|
|
catalytic cracking |
Conditions: Low pressure High Temperature (450°C) Zeolite Catalyst
Produces branched and cyclic alkanes and Aromatic hydrocarbons used foe making motor fuels Branched and cyclic hydrocarbons burn more cleanly and are used to give fuels a higher octane number cheaper then thermal cracking as it saves energy as lower temp and pressures are used |
|
|
combustion fuel |
releases heat energy when burnt alkanes Alkanes readily burn in thepresence of oxygen. Thiscombustion of alkanes is highlyexothermic, explaining their useas fuels. |
|
|
complete combustion |
In excess oxygen alkanes will burn with complete combustionThe products of complete combustion are CO2and H2O.
C8H18(g) + 12.5 O2(g) → 8CO2(g) + 9 H2O(l) |
|
|
incomplete combustion |
If there is a limited amount of oxygen then incomplete combustionoccurs, producing CO (which is very toxic) and/or C (producing a sootyflame)
CH4(g) + 3/2 O2(g) → CO(g) + 2 H2O(l) CH4(g) + O2(g) → C(s) + 2 H2O(l) Incomplete combustion producesless energy per mole thancomplete combustion |
|
|
pollution from combustion |
Carbon (soot) can cause globaldimming- reflection of the sun’slight
|
|
|
sulphur pollution |
Sulphur containing impurities are found in petroleum fractions whichproduce SO2 when they are burned. Coal is high in sulphur content, andlarge amounts of sulphur oxides areemitted from power stations. S+ O2 SO2 CH3SH+ 3O2 SO2 + CO2 + 2H2OSO2 will dissolve in atmospheric water and can produce acid rain.
|
|
|
sulphur use and removal |
SO2can be removed from the waste gases from furnaces (e.g. coal firedpower stations) by flue gas desulphurisation. The gases pass through ascrubber containing basic calcium oxide which reacts with the acidicsulphur dioxide in a neutralisation reaction
SO2 + CaO CaSO3 The calcium sulphite which isformed can be used to makecalcium sulphate forplasterboard. |
|
|
Nitrogen Oxides NOx
|
Nitrogen oxides form from the reaction between N2 and O2 inside the car engine. The high temperature and spark in the engine provides sufficient energy to break strong N2 bond
N2 + O2 2NO N2 + 2O2 2NO2 |
|
|
pollutant and enviromental consequence |

|
|
|
Catalytic converters
|
These remove CO, NOx and unburned hydrocarbons (e.g. octane, C8H18) from the exhaust gases, turning them into ‘harmless’ CO2 , N2 and H2O. 2 CO + 2 NO → 2 CO2 + N2 C8H18 + 25 NO → 8 CO2 + 12½ N2 + 9 H2O
Converters have a ceramichoneycomb coated with a thinlayer of catalyst metalsPlatinum, Palladium, Rhodium– to give a large surface area. |
|
|
global warming |
•Carbon dioxide (CO2 ), methane (CH4 ) and water vapour (H2O) are all greenhouse gases. (They trap the Earth’s radiated infra red energy in the atmosphere). •Water is the main greenhouse gas (but is natural), followed by carbon dioxide and methane
|
|
|
carbon dioxide increase reason and consequence |
Carbon dioxide levels have risen significantly in recent years due to increasing burning of fossil fuels. Carbon dioxide is a particularly effective greenhouse gas and its increase is thought to be largely responsible for global warming
The Earth is thought to be getting warmer, and many scientists believe it is due to increasing amounts of greenhouse gases in the atmosphere |
|
|
chem golbal warming disagreement |
Some scientists disagree and think the sun’s activity is largely responsible for climatic variations
|

