![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
What kinds of chemical mediator for we have in the CNS?
|
❤Neurotransmitters:
-released by presynaptic terminals & produce rapid excitatory responses in post-synaptic neurons -can be divided into fast neurotransmistters eg. glutamate, glycine, GABA, ACh that operate through ligand gated ion channes and slow neurotransmitters & neuromodlators e.g. dopamine, 5HT, ACh, neuropeptides that operate though G-protein coupled receptors -the time scale of the response to the transmitter depends on the nature of the receptor to which it binds ❤Neuromodulators -released by neurons, and produce slower pre- or post-synaptic responses, mediated mainly by G-protein coupled receptors ❤Neurotrophic factors: -released mainly by non-neuronal cells, act on tyrosine-kinase-linked receptors that regulate gene expression |
|
|
What are the stimulatory neurotransmitters in the CNS?
|
❤Glutamate is the principle fast "classical" excitatory transmitter & is widespread through the CNS
-binds to NMDA, AMPA, kainate receptor etc. |
|
|
What are the inhibitory neurotransmitters in the CNS?
|
❤Gamma-amino butyric acid (GABA) is the major inhibitoryneurotransmitter
-2 types: GABA-A which is ligand gated to Cl- ion channels and GABA-B receptor is G-protein-coupled receptor, coupled to biohemical processes and regulation of ion channels ❤Glycine is an inhibitory transmitter acting mainly in the spinal cord ❤Acetylcholine is widely distributed in the brain -both nicotinic and muscarinic receptors occur in the CNS -muscarinic receptors mediate the main behavioral effects associated with ACh- arousal level, learning and short term memory |
|
|
What are the monoamines found in the CNS?
|
❤Dopamine - neurotransmitter & precursor of NE
-2 main families: D1 & D2 ❤Norepinephrine - adrenergic receptors are α1, α2 & β -important in the "arousal" system, controlling wakefulness, blood pressure regulation & control of mood ❤5-hydroxytryptamine (serotonin) - important CNS transmitter with complex and varied effects -can exert excitatory or inhibitory effects, acting presynaptically or post-synaptically -5-HT pathways are involved in physiological and behavioural funcions, namely hallucinations, and behavior changes, sleep, wakefulness and mood, control of sensory transmission |
|
|
What non-adrenergic non-cholinergic (NANC) transmitters do we have?
|
❤Histamine - H1, H2, H3 are widespread in the brain
-function not clear, but H1-receptor antagonists are strongly sedative and anti-emetic ❤Purines (ATP & adenosine), nitric oxide, and arachidonic acid are also transmitters and modulators |
|
|
What neuropeptides act in the CNS?
|
❤Opiod peptides that modulate pain pathways
-substance P and neuropeptide Y |
|
|
What are the classes of analgesics are used in veterinary medicine?
|
Local anaesthetics, Non-steroidal anti-inflammatory drugs, opioid analgesitcs, centrally acting non-opiod analgesics, alpha 2 adrenergic agonists
|
|
|
Describe the pathway for nociception
|
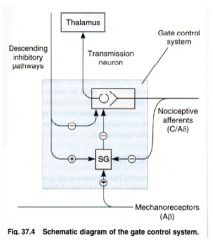
❤Nociceptive afferent
-2 types: 1) C-fibers that are non-myelinated, have a lot conduction velocity, and sense dull pain 2) A-fibers that are fine myelinated, and have faster conduction velocities -sense sharp localized pain ❤GATE-CONTROL MECHANISM in the Dorsal Horn -this center modulates pain transmission -inhibitory interneurons in the substantia gelatinosa of the dorsal horn act to inhibit the transmission pathway Inhibitory interneurons are activated by descending pathways from the mid-brain and brainstem, as well as non-nociceptive afferent input Inhibitory interneurons are inhibited by persistent C-fiber activity, hence 'wind up' phenomenon - increasing duration of stimulation leads to increased transmission of pain signals ❤Descending inhibitory controls: pain modulation -2 areas of the brain, in the mid-brain/pons 'peri aqueductal grey area' and in the lower pons/medulla 'nucleus raphe magnus' initiate descending pathways that exert a strong inhibitory effect on the dorsal horn -this descending inhibition is mediated by enkephalins, 5-HT, noradrenaline and adenosine ❤Application of a cold, warm, or touch sitimulates the A-beta non-nociceptive dorsal root fibers -the A-beta non-nociceptive dorsal root fibers counteract the pain by stimulating the projection neuron or by stimulating an inhibitory interneuron ❤Endogenous opioids are major inhibitory neurotransmitters in nociceptive pathways -most important are β-endorphin, enkephalins, and dynorphin |
|
|
What are the opioid receptors?
|
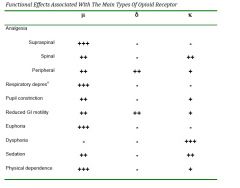
❤mu-receptors (µ) - µ1: mediate most analgesic effects, euphoria, supraspinal
-enkephalins are endogenous µ1 liganss -µ2 mediate most of the undesirable side effect such as respiratory depression, constipation ❤Delta receptors (δ) - are important in the periphery where they contribute to analgesia ❤Kappa receptors (κ) mediated analgesia primarily at spinal cord level and have fewer side effects - less respiratory depression, mioisis etc -they tend to cause sedation and dysphoria rather than euphoria |
|
|
What are endorphins? What is an opioid?
|
Naturally occurring (endogenous) peptides are called endorphins
An opioid is any substance, whether endogenous or synthetic, that produces morphine-like effects that are blocked by antagonists such as naloxone |
|
|
What are pure opioid aganosists? Mixed agonist-antagonists? Partial agonists?
|
❤Pure agonists have high affinity for the µ receptor
-includes most of the morphine like drug eg. morphine, pethidine, fentanyl Mixed Agonist-antagonists and partial agonists -combine agonist effect on one receptor subtype with antagonist activity on another receptor type -butorphanol is an antagonist at the µ-receptor, and an agonist κ-receptor; pentzocine - is a weak antagonist, and κ agonist; -buprenorphine is a partial µ-recptor agonosit (it has a high affinity for the receptor but only partial activity) |
|
|
What are opioid antagonists used for?
|
-used to treat overdoses to opiods
|
|
|
What are the cellular actions of opioids?
|
-opioid receptors are linked to G-proteins
-close voltage-gated Ca2+ channels on pre-synaptic terminals, and thereby reduce transmitter release -open K+ channels on post-synaptic neurons, thereby causing hyperpolarization, and inhibition of action potential in post synaptic neurons |
|
|
What are the sites of action of opioid drugs?
|
-dorsal horn of spinal cord, so powerful analgesic effect direct on spinal cord
-supraspinal sites - pain modulating descending pathways -opioids directly inhibit neurons -opioids activate neurons that inhibit pain transmission -exogenous opioids stimulate release of endogenous opioid peptides, that have actions at δ and κ receptors -peripheral opioid action - at sites outside CNS, particular in pain associated with inflammation |
|
|
What actions do opioids have on the central nervous system?
|
-analgesia (µ1 mediated), reduces nociception at periaqueductal gray level at spinal level; reduces stress at limbic system
-euphoria (µ2 mediated), contentment and well being; balanced by κ-mediated dysphoria -excitement and constant motor response, & convulsions. Seen more often in domestic animals particularly horses & cats, than in humans. Presumed µ mediated as seen with morphine and pethidine but not agonist-antagonists such as butorphanol -respiratory depression (µ2 mediated), decreased sensitivity of respiratory centre to pCO2 that occurs at therapeutic doses -depression of coughing reflex, mechanism uncertain. Codeine gives cough suppression in subanalgesic doses -nausea and vomiting (µ2 mediated) through activation at chemoreceptor trigger zone -pupillary constriction - centrally mediated via oculomotor nucleus, important emergency diagnostic in humans. Marked species variability |
|
|
What actions do opioids have on the gastrointestinal system?
|
-increased tone and reduced motility (µ, κ, and δ mediated) leading to constipation, and delayed gastric emptying
|
|
|
What other actions do opioids have?
|
-morphine stimulates release of histamine from mast cells which can cause uticaria, bronchoconstriction, hypotension
-bradycardia and hypotension with high doses through a direct effect on medulla |
|
|
What can happen with use of opioids and how can this be reversed?
|
-TOLERANCE develops rapidly, accompanied by physical withdrawal syndrome
-dependence is satisfied by µ receptor agonists, and withdrawal is precipitated by µ-receptor antagonists -methadone is a weak, long acting µ-receptor agonist used to relieve withdrawal symptoms -some opioid analgesics e.g. codeine, buprenorphine, butorphanol are much less likely to cause dependence -because of their dependence characteristics ALL opioids are Schedule 8 poisons |
|
|
Describe the pharmacokinetics of opioids
|
-administered by injection
-metabolised in liver by conjugation with glucoronide -these glucoronides may be pharmacologically active |
|
|
What are the opioid drugs?
|
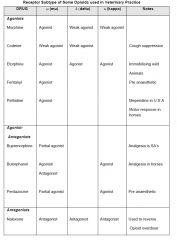
|

