![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
Transition elements (properties) Define transition element |
A d-block element that forms an ion with an incomplete d subshell |
|
|
Transition elements (properties) Why are Scandium and Zinc not transition elements? |
They do not form ions with incomplete d-sub shells Sc forms Sc 3+ - in which d-orbitals are empty Zn forms Zn 2+ - in which d-orbitals are full |
|
|
Transition elements (properties) Draw the diagram cheat thing for the energy levels of orbitals (Aufbau principle) |
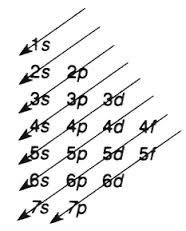
|
|
|
Transition elements (properties) How do Chromium and Copper differ from other transition elements? |
They do not follow the Aufbau principle: Chromium - the 3d and 4s orbitals all contain one electron with no orbital completely filled Copper - the 3d orbitals are full, but there is only one electron in the 4s orbital |
|
|
Transition elements (properties) Draw the entire electronic configurations for transition elements. |
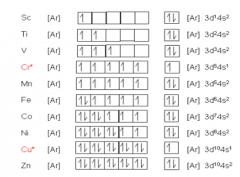
|
|
|
Transition elements (properties) Why do Copper and Chromium not follow this pattern? |
electron repulsions between the outer electrons are minimised, resulting in increased stability of the atoms |
|
|
Transition elements (properties) In their reactions, transition element atoms... |
lose ions to form positive ions. |
|
|
Transition elements (properties) Where are electrons lost from first? |
4s electrons are lost first despite them being filled first because once occupied, 4s electrons have a higher energy level. |
|
|
Transition elements (properties) Describe the physical properties of transition metals. |
Normal metallic properties: shiny, high densities, high melting points and high boiling points. When solid, they exist as giant metallic lattices containing delocalised electrons which move to conduct electricity. |
|
|
Transition elements (properties) Use of Ni? |
Alloyed with copper for use in making 'silver' coins |
|
|
Transition elements (properties) Use of titanium |
Component for joint replacement parts - ball + socket for hip replacement |
|
|
Transition elements (properties) Use of Iron? |
Cast to make telephone boxes and post boxes, or alloyed for use in the construction of buildings and bridges. |
|
|
Transition elements (properties) What do transition elements form? |
Compounds in which the transition metal has different oxidation states.
|
|
|
Transition elements (properties) What do transition element compounds form? and often do? |
Coloured solutions when dissolved in water , they often catalyse chemical reactions. |
|
|
Transition elements (properties) The transition elements all form compounds... |
with ions in a +2 oxidation state by the loss of two electrons in the 4s orbital.
|
|
|
Transition elements (properties) Why are 4s electrons lost first? |
When electrons occupy the 4s orbital it has a higher energy level. |
|
|
Transition elements (properties) What do strong oxidising agents often contain? Give two examples. |
The highest oxidation state of a transition element. Manganese forms potassium permanganate, KMnO4, a purple solid used as an oxidising agent in redox titrations. Mn - 7+ |
|
|
Transition elements (properties) How are transition metal ions coloured? |
When white light passes through a solution containing transition metal ions, some of the wavelengths of visible light are absorbed. The colour we observe is a mixture of the wavelengths of light that are not absorbed. |
|
|
Transition elements (properties) Describe the colour of Copper (II) sulfate |
Appears pale blue because the solution absorbs the red/orange region of the electromagnetic spectrum and reflects/transmits the blue. |
|
|
Transition elements (properties) What is colour in organic chemistry linked to? |
The partially filled d-orbitals of transition metal ions. |
|
|
Transition elements (properties) Describe the colour of Scandium(III). Include the electronic configuration., |
No colour - there are no partially filled d-orbitals. 1s2 2s2 2p6 3s2 3p6 |
|
|
Transition elements (properties) Define catalyst |
A substance that increases the rate of a chemical reaction by providing an alternative route for the reaction to follow with lower activation energy. |
|
|
Transition elements (properties) Why do transition metals act as catalysts? |
1) Transition metals provide a surface on which a reaction can take place. Reactants are adsorbed onto the surface of the metal and held in place while a reaction occurs. After the reaction, the products are desorbed and the metal remains unchanged. 2) Transition metal ions have the ability to change oxidation states by gaining or losing electrons. They bind to reactants forming intermediates as part of a chemical pathway with lower activation energy. |
|
|
Transition elements (properties) What are the four processes catalysed by transition metals ? |
1) Haber process 2) Contact process 3) Hydrogenation of alkenes 4) Decomposition of Hydrogen Peroxide |
|
|
Transition elements (properties) Describe the Haber process. Include catalyst and catalyst function. |
Used to make ammonia, NH3, from N2 and H2. N2 (g) + 3H2 (g) <---> 2NH3(g) Catalyst: Iron metal. Increases rate, lowers temp at which reaction takes place. |
|
|
Transition elements (properties) Describe the Contact process. Include the catalyst |
Used to convert SO2 sulfur dioxide into SO3 sulfur trioxide, (for the manufacture of H2SO4) 2SO2 (g) + O2 (g) <---> 2SO3(g) Catalyst : Vanadium(V) oxide, V2O5 = +5 H2SO4 is an important inorganic chemical with many uses: production of... -fertilisers -detergents -adhesives and explosives -electrolyte in car batteries |
|
|
Transition elements (properties) Describe hydrogenation of alkenes. Include catalyst. |
Hydrogen is added across the C=C double bonds in unsaturated compounds to form saturated compounds - this is HYDROGENATION. C2H4 + H2 ---> C2H6 Catalyst: Ni - lowers temp and pressure required. - Used in the hydration of unsaturated vegetable oils to make spreadable margarine |
|
|
Transition elements (properties) Describe the decomposition of hydrogen perocide. |
Hydrogen peroxide decomposes slowly at room temperature and pressure into H2O and O2. A catalyst is added to increase reaction rate. 2H2O2 (g) ---> 2H2O (l) + O2 (g) Catalyst: Manganese(IV) oxide, MnO2 = +4. Used to produce O2 (g) |
|
|
Transition elements (precipitation reactions) What is a precipitation reaction? |
A reaction in which soluble ions in separate solutions are mixed together to produce an insoluble compound which settles out of solution as a solid (this is the ppt) |
|
|
Transition elements (precipitation reactions) How do transition metal ions in aqueous solution form precipitates? |
Transition metal ions in aqueous solution react with aq NaOH to form coloured precipitates. |
|
|
Transition elements (precipitation reactions) Draw a table to show the reactions of Cu2+, Co2+, Fe2+ and Fe3+ with aq NaOH. Include relevant equations, and colour change that occurs. |

|
|
|
Transition elements (ligands and complex ions) |
h |

