![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
39 Cards in this Set
- Front
- Back
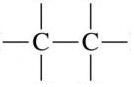
|
Alkane
|
|
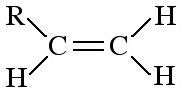
|
Alkene
Alkenes consist of a C=C double bond function. Alkenes can be shown in text as: Mono substituted: RCH=CH2 1,1-disubstituted: R2C=CH2 1,2-disubstituted: RCH=CHR Alkanes are planar as there is no rotation about the C=C bond. Alkenes are electron rich reactive centres and are susceptible to electrophilic addition. |
|
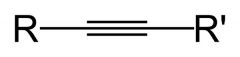
|
Alkyne
|
|
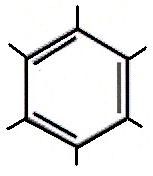
|
Arene
(Aromatic Ring) |
|
|
R
|
Alkane
Alkyl, and occasionally aryl (aromatic) functions are represented by the R- Methyl: CH3– Ethyl: CH3CH2– Propyl: CH3CH2CH2– Isopropyl: (CH3)2CH– Phenyl: C6H5– etc. |
|
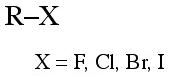
|
Alkyl halide
Alkyl halides [haloalkanes] consist of an alkyl group attached to a halogen: F, Cl, Br, I. Chloro, bromo and iodo alkyl halides are often susceptible to elimination and/or nucleophilic substitution reactions. |
|
|
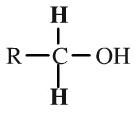
Primary Alcohol
Primary alcohols have an -OH function attached to an R-CH2- group. Primary alcohols can be oxidised to aldehydes and on to carboxylic acids. (It can be difficult to stop the oxidation at the aldehyde stage.) Primary alcohols can be shown in text as: RCH2OH |
|
|
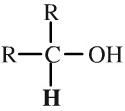
Secondary Alcohol
Secondary alcohols have an -OH function attached to a R2CH- group. Secondary alcohols can be oxidised to ketones. Secondary alcohols can be shown in text as: R2CHOH |
|
|
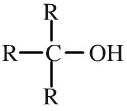
Tertiary Alcohol
Tertiary alcohols have an -OH function attached to a R3C- group. Tertiary alcohols are resistant to oxidation with acidified potassium dichromate(VI), K. Tertiary alcohols can be shown in text as: R3COH |
|
|
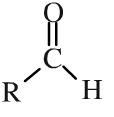
Aldehyde
Aldehydes have a hydrogen and an alkyl (or aromatic) group attached to a carbonyl function. Aldehydes can be shown in text as: RCHO Aldehydes are easily oxidised to carboxylic acids, and they can be reduced to primary alcohols. |
|
|
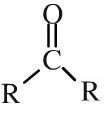
Keytone
Ketones have a pair of alkyl or aromatic groups attached to a carbonyl function. Ketones can be shown in text as: RCOR |
|
|
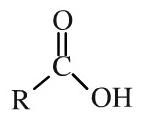
Carboxylic acid
Carboxylic acids have an alkyl or aromatic groups attached to a hydroxy-carbonyl function. Carboxylic acids can be shown in text as: RCOOH Carboxylic acids are weak Bronsted acids and they liberate CO2 from carbonates and hydrogen carbonates. |
|

|
Carbonyl function
The carbonyl group is a super function because many common functional groups are based on a carbonyl, including: aldehydes, ketones, carboxylic acids, esters, amides, acyl (acid) chlorides, acid anhydrides |
|
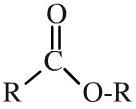
Ester
|
Ester
Esters have a pair of alkyl or aromatic groups attached to a carbonyl + linking oxygen function. Esters can be shown in text as: RCOOR or (occasionally) ROCOR. carboxylic acid + alcohol -> ester + water This is an acid catalysed equilibrium |
|
|
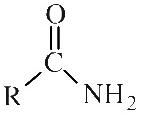
Amide
Primary amides (shown) have an alkyl or aromatic group attached to an amino-carbonyl function. Primary amides can be shown in text as: RCONH2 Secondary amides have an alkyl or aryl group attached to the nitrogen: RCONHR Tertiary amides have two alkyl or aryl group attached to the nitrogen: RCONR2 |
|
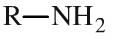
|
Primary amine
Primary amines have an alkyl or aromatic group and two hydrogens attached to a nitrogen atom. Primary amines can be shown in text as: RNH2 Primary amines are basic functions that can be protonated to the corresponding ammonium ion. Primary amines are also nucleophilic |
|
|
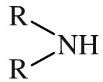
Secondary amine
Secondary amines have a pair of alkyl or aromatic groups, and a hydrogen, attached to a nitrogen atom. Secondary amines can be shown in text as: R2NH Secondary amines are basic functions that can be protonated to the corresponding ammonium ion. Secondary amines are also nucleophilic. |
|
|
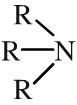
Tertiary amine
Tertiary amines have three alkyl or aromatic groups attached to a nitrogen atom. Tertiary amines can be shown in text as: R3N Tertiary amines are basic functions that can be protonated to the corresponding ammonium ion. Tertiary amines are also nucleophilic |
|
|
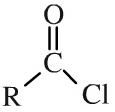
Acid chloride
Acid chlorides, or acyl chlorides, have an alkyl (or aromatic) group attached to a carbonyl function plus a labile (easily displaced) chlorine. Acid chlorides highly reactive entities are highly susceptible to attack by nucleophiles. Acid chlorides can be shown in text as: ROCl |
|
|
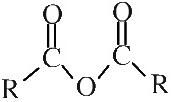
Acid anhydride
(carboxillic) acid anhydride Acid anhydrides are formed when water is removed from a carboxylic acid, hence the name. Acid anhydrides can be shown in text as: (RO)2O |
|
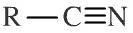
|
Nitrile
Nitriles (or organo cyanides) have an alkyl (or aromatic) group attached to a carbon-triple-bond-nitrogen function. Nitriles can be shown in text as: RCN Note that there is a nomenclature issue with nitriles/cyanides. If a compound is named as the nitrile then the nitrile carbon is counted and included, but when the compound is named as the cyanide it is not. For example: CH3CH2CN is called propane nitrile or ethyl cyanide (cyanoethane). |
|
|
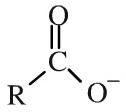
Carboxylate ion or salt
Carboxylate ions are the conjugate bases of carboxylic acids, ie. the deprotonated carboxylic acid. Carboxylate ions can be shown in text as: RCOO– When the counter ion is included, the salt is being shown. Salts can be shown in text as: RCOONa |
|
|
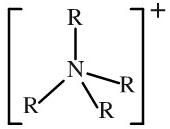
Ammonium ion
Ammonium ions have a total of four alkyl and/or hydrogen functions attached to a nitrogen atom. [NH4]+ [RNH3]+ [R2NH2]+ [R3NH]+ [R4N]+ Quaternary ammonium ions are not proton donors, but the others are weak Bronsted acids (pKa about 10). |
|
|
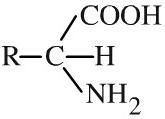
Amino acid
Amino acids, strictly alpha-amino acids, have carboxylic acid, amino function and a hydrogen attached to a the same carbon atom. There are 20 naturally occurring amino acids. All except glycine (R = H) are chiral and only the L enantiomer is found in nature. Amino acids can be shown in text as: R-CH(NH2)COOH |
|
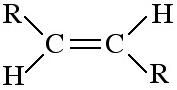
|
trans-Alkene
trans-alkenes are 1,2-disubstituted functions with the two R, X or other groups on opposite sides of the C=C function. Due to the non-rotation of the C=C bond, cis and trans geometric isomers are not [thermally] Interconverted. |
|
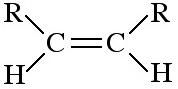
|
cis-Alkene
cis-Alkenes are 1,2-disubstituted functions with the two R, X or other groups on the same side of the C=C function. Due to the non-rotation of the C=C bond, cis and trans geometric isomers are not [thermally] Interconvertion. |
|
|
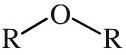
Ether
Ethers have a pair of alkyl or aromatic groups attached to a linking oxygen atom. Ethers can be shown in text as: ROR Ethers are surprisingly unreactive and are very useful as solvents for many many (but not all) classes of reaction. |
|
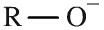
|
Alkoxide ion
Alkoxide ions an alkyl group attached to an oxyanion. Alkoxide ions can be shown in text as: RO– Sodium alkoxides, RONa, are slightly stronger bases than water and so cannot be prepared in water. Instead they are prepared by adding sodium to the dry alcohol. |
|
|
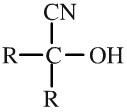
Hydroxynitrile
Hydroxynitriles (also called cyanohydrins) are formed when hydrogen cyanide, H+ CN–, adds across the carbonyl function of an aldehyde or ketone |
|
|
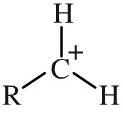
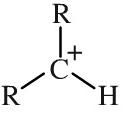
Primary carbocation
Primary carbocations have a single alkyl function attached to a carbon centre with a formal positive charge. Carbocations - also and more correctly called carbenium ions - are important reactive intermediates implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Stability: primary << secondary << tertiary |
|
|
Secondary carbocation
Secondary carbocations have a pair of alkyl functions attached to a carbon centre with a formal positive charge. Carbocations - also and more correctly called carbenium ions - are important reactive intermediates implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Stability: primary << secondary << tertiary |
|
|
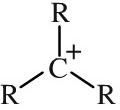
Tertiary carbocation
Tertiary carbocations have three alkyl functions attached to a carbon centre with a formal positive charge. Carbocations - also and more correctly called carbenium ions - are important reactive intermediates implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Stability: primary << secondary << tertiary |
|
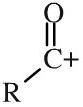
|
Acyl cation
Acyl cations have an alkyl (or aromatic) group attached to a carbonyl function with a formal positive charge. Acyl cations are important reactive intermediates and are implicated in electrophilic addition reactions and electrophilic aromatic substitution reactions. Acyl cations are commonly formed from the corresponding acyl/acid chloride plus aluminium chloride. |
|
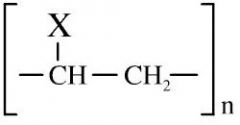
|
Polymer
Polymers consist of small monomer molecules that have reacted together so as to form a large covalently bonded structure. There are two general types of polymerisation: addition and condensation. Linear chain polymers are generally thermoplastic, while three dimensional network polymers are not |
|
|
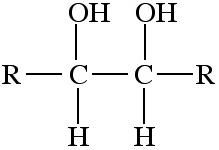
Diol or polyol
Diols and polyols are alcohols with two or more -OH functions. Diols and polyols are very soluble in water. They are used as high temperature polar solvents. |
|
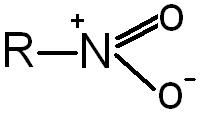
|
Nitro
|
|
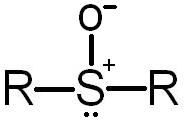
|
Sulfoxide
|
|
|
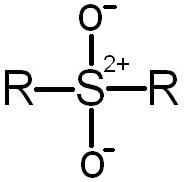
Sulfone
|
|
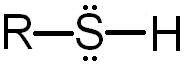
|
Thiol
|

