![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
52 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What Are Fats?
|
Fats are one type of lipid.
|
|
|
|
Lipids
|
diverse class of molecules that are insoluble in water.
Lipids (fats) do not dissolve in water |
Fats are one type of lipid. The term fat is a synonym for triglyceride, which is a molecule composed of glycerol with three fatty acids attached to it. That is the type of lipid we store on our bodies, for instance, around our waistline. It is a hydrophobic molecule that will not dissolve in water.
|
|
|
The Three types of lipids that are found in foods
|
Triglycerides
Phospholipids Sterols |
The three types of lipids found in foods are triglycerides, phospholipids, and sterols. As a class, lipids are generally nonpolar, hydrophobic, and will not dissolve in water. It was mentioned on the last slide that fat and triglyceride mean the same thing. Triglycerides are great energy storage molecules. Phospholipids are slightly different than triglycerides because the phosphate portion of them is polar and hydrophilic while the lipid tails are nonpolar and hydrophobic. This makes them unique in the lipid family because they have both hydrophilic and hydrophobic properties. Molecules that have both water-soluble and water-insoluble characteristics are termed amphipathic. Phospholipids function primarily as membrane components. Sterols are exemplified by cholesterol, the so-called base steroid. That is because many important compounds are made out of cholesterol. For instance, the sex hormones are modified cholesterol. That includes estrogen, progesterone, and testosterone
|
|
|
Triglycerides
|
are composed of
-Three fatty acid molecules -One glycerol molecule |
A triglyceride is made of three fatty acids attached to the 3-carbon alcohol, glycerol. The fatty acids can be of varying chain length. Each fatty acid is composed of carbon atoms surrounded by hydrogen atoms with a carboxyl group at one end. A carboxyl group is –COOH. The oxygen in the carboxyl group is the only oxygen in the fatty acid molecule. The presence of a lot of carbons surrounded by hydrogen atoms makes the molecule nonpolar and hydrophobic. It is unable to form hydrogen bonds with water molecules, which is why it won’t dissolve in water. Basically, when it contacts water, it tries to get away from it, and being less dense than water, triglycerides will float on top of it. We all know that if we pour vegetable oil into a glass of water, the oil and water will not mix and the oil, which contains triglycerides, will float on top.
|
|
|
Fatty acids
|
long chains of carbon atoms surrounded by hydrogen atoms (hydrocarbons)
|
|
|
|
Glycerol
|
a 3-carbon alcohol that is the backbone of a triglyceride
|
|
|
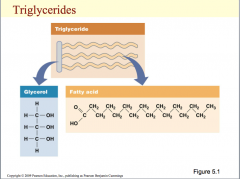
Here is an illustration of a triglyceride. The structure of the 3-carbon alcohol, glycerol, is shown as having 3 carbon atoms with hydrogen atoms on one side and at the top and bottom, and on the other side of the carbon atoms are three hydroxyl groups. A hydroxyl group contains –OH. Each fatty acid is attached through a dehydration synthesis reaction in which water is removed to form a bond. The hydrogen from the hydroxyl group of glycerol is removed along with a hydrogen and oxygen from the carboxyl group on the fatty acid producing water and forming the bond. At the top of the slide, a triglyceride is shown as a bar with three wavy lines attached to it. The bar is glycerol and the three wavy lines represent fatty acids.
|
Fatty acids can differ in
Length of their carbon chain Short- (<6), medium- (6–12), or long- (>12) chain Chain length determines how fatty acids are digested and absorbed Short- and medium-chain fatty acids are digested and transported more quickly than long-chain fatty acids Level of saturation Saturation refers to how many hydrogen atoms surround each carbon Shape |
Fatty acids can differ in the length of their carbon chains, their level of saturation, & their shape. Short-chain fatty acids contain less than 6 carbon atoms. Medium-chain fatty acids have from 6-12 carbon atoms, & long-chain fatty acids have more than 12 carbon atoms in their carbon chain. The longer the fatty acid, the less water-soluble it is. Since blood is mostly water, long-chain fatty acids can’t enter it after being absorbed in the small intestine. They go into the lymph system. Short & medium chain fatty acids can be absorbed and transported into the blood by a protein called albumin, which takes them directly to the liver for processing. Some fatty acids have no double bonds between the carbon atoms in the carbon chain, a condition referred to as saturated. A double bond between 2 carbon atoms in the chain is called a point of unsaturation.
|
|
|
Triglyceride (continued from picture side 3)
|
The amount of saturation affects the shape of the triglyceride. Saturated fatty acids are straight while unsaturated fatty acids bend or kink around the double bond.
|
|
|
|
Saturation
|
Saturation refers to how many hydrogen atoms surround each carbon
|
|
|
|
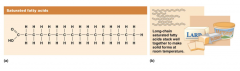
Saturated fatty acids
|
have hydrogen atoms surrounding every carbon in the chain. No double bonds.
|
The expression “saturated” comes from the idea that if there are all single bonds between the carbon atoms in a fatty acid chain, then the maximum number of hydrogen atoms can be attached to the carbons. The carbon chain is saturated with hydrogens. If a double bond is formed between two of the carbons in the fatty acid chain, then there will be two less hydrogen atoms attached to those two carbon atoms than would be possible with a single bond between the carbon atoms instead, so the fatty acid is said to be unsaturated. The easiest way to remember the meaning of saturated is simply to know that all of the bonds between the carbon atoms in the fatty acid chain are single bonds. If the fatty acid chain has one double bond between two carbon atoms, then it is called monounsaturated, and if it has two or more double bonds, it is referred to as polyunsaturated.
|
|
|
Monounsaturated fatty acids
|
lack hydrogen atoms in one region. One double bond.
Note: Each double bond causes the loss of two hydrogen atoms. |
The expression “saturated” comes from the idea that if there are all single bonds between the carbon atoms in a fatty acid chain, then the maximum number of hydrogen atoms can be attached to the carbons. The carbon chain is saturated with hydrogens. If a double bond is formed between two of the carbons in the fatty acid chain, then there will be two less hydrogen atoms attached to those two carbon atoms than would be possible with a single bond between the carbon atoms instead, so the fatty acid is said to be unsaturated. The easiest way to remember the meaning of saturated is simply to know that all of the bonds between the carbon atoms in the fatty acid chain are single bonds. If the fatty acid chain has one double bond between two carbon atoms, then it is called monounsaturated, and if it has two or more double bonds, it is referred to as polyunsaturated.
|
|
|
Polyunsaturated fatty acids
|
lack hydrogen atoms in multiple locations. Two or more double bonds.
Note: Each double bond causes the loss of two hydrogen atoms. |
The expression “saturated” comes from the idea that if there are all single bonds between the carbon atoms in a fatty acid chain, then the maximum number of hydrogen atoms can be attached to the carbons. The carbon chain is saturated with hydrogens. If a double bond is formed between two of the carbons in the fatty acid chain, then there will be two less hydrogen atoms attached to those two carbon atoms than would be possible with a single bond between the carbon atoms instead, so the fatty acid is said to be unsaturated. The easiest way to remember the meaning of saturated is simply to know that all of the bonds between the carbon atoms in the fatty acid chain are single bonds. If the fatty acid chain has one double bond between two carbon atoms, then it is called monounsaturated, and if it has two or more double bonds, it is referred to as polyunsaturated.
|
|
The expression “saturated” comes from the idea that if there are all single bonds between the carbon atoms in a fatty acid chain, then the maximum number of hydrogen atoms can be attached to the carbons. The carbon chain is saturated with hydrogens. If a double bond is formed between two of the carbons in the fatty acid chain, then there will be two less hydrogen atoms attached to those two carbon atoms than would be possible with a single bond between the carbon atoms instead, so the fatty acid is said to be unsaturated. The easiest way to remember the meaning of saturated is simply to know that all of the bonds between the carbon atoms in the fatty acid chain are single bonds. If the fatty acid chain has one double bond between two carbon atoms, then it is called monounsaturated, and if it has two or more double bonds, it is referred to as polyunsaturated.
|
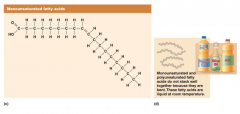
In contrast to saturated fatty acids, unsaturated fats will bend around the double bond. This doesn’t allow them to be stacked one right on top of the other and results in them being liquid at room temperature. Vegetables typically have more unsaturated fatty acids than animal products and are often found as oils. The fatty acid shown on this slide is an 18-carbon monounsaturated fatty acid found in large amounts in olive and canola oils called oleic acid.
|
|
|
|
The shape of a triglyceride
|
The shape of a triglyceride is determined by the saturation of the carbon chains.
Saturated fatty acids can pack tightly together and are solid at room temperature. For example, coconut oil, animal fats, butter, and lard are high in saturated fatty acids |
A food that has a lot of saturated fatty acids in it will be solid at room temperature because the linear chains can be stacked tightly on top of one another. Animal fats such as butter and lard behave this way. Some, but not very many vegetable products are also high in saturated fats. Palm oil and coconut oil are examples. However, the saturated fatty acids in these vegetable oils are short-chain fatty acids whereas the saturated fats in animal products are usually 16 to 18 carbons long.
|
|
|
Triglycerides continued
|
Unsaturated fatty acids do not stack together well and are liquid at room temperature.
Unsaturated fatty acids are the predominant type in plants Two exceptions are coconut and palm kernel oil The hydrogen atoms at the unsaturated region can be arranged in different positions. Cis: same side of the carbon chain Trans: opposite sides of the chain |
Since a fatty acid will bend around a double bond between two of the carbons, unsaturated fatty acids will not pack together tightly causing them to remain liquid at room temperature. Unsaturated fatty acids are found predominantly in plants with the exception of the aforementioned palm and coconut oils. Where there is a double bond between two carbon atoms, only two hydrogen atoms can be attached to those carbon atoms. In nature, the hydrogens are always on the same side of the carbon atoms and not across from each other. Being on the same side is called the cis conformation and being across from each other is called the trans conformation. Only the cis conformation occurs naturally. The trans conformation in some of our foods is the result of human food processing.
|
|
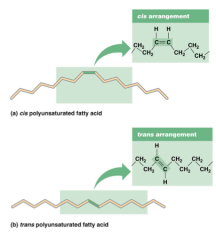
The drawing on this slide shows the cis arrangement towards the top of the slide. Both of the hydrogen atoms that are attached to the two carbon atoms sharing a double bond are on the same side, in this case, both above the carbons. This is the configuration we see in natural foods. At the bottom of the slide is the trans conformation in which one hydrogen atom is on the top of the carbon chain and the other is across from it on the bottom of the carbon chain. This arrangement occurs due to food processing. It is the result of hydrogenation of fatty acids to make food last longer on the shelf in markets.
|
.
|
.
|
|
|
Hydrogenation
|
the addition of hydrogen atoms to unsaturated fatty acids.
Converts liquid fats (oils) into a semi-solid (spreadable) or solid form Used to create margarine from plant oil Often creates trans fatty acids Listed on the food label as partially hydrogenated oil |
Hydrogenation refers to the addition of hydrogen to a fatty acid to get rid of double bonds and convert an unsaturated fatty acid to a saturated one. It turns out that double bonds are susceptible to oxidative stress, which means they will spoil and turn rancid. If you’ve ever tasted a food that’s rancid, you know it’s not a very pleasant flavor. Saturated fats are not oxidized as easily, so foods that have high amounts of them will not spoil very quickly. When this was recognized by the food industry, they came up with the idea of taking items such as peanut butter, which has a lot of unsaturated fatty acids, and getting rid of the double bonds while adding hydrogen to saturate the fats. By doing this, they were able to extend the shelf life of peanut butter in the supermarket.
|
|
|
Hydrogenation continued
|
continued from side 3: . At first, they completely hydrogenated the foods, but researchers investigating heart disease discovered that saturated fats might contribute to the malady, so food manufacturers adjusted by only partially hydrogenating the foods. “Partially hydrogenated” is what you often see on a food label. The problem is that the process of hydrogenation causes the hydrogen atoms around many of the double bonds that remain to switch from the cis arrangement to the trans arrangement. Research has shown that the trans configuration in fatty acids is even more harmful than saturated fatty acids in terms of cardiovascular disease. Therefore, nutritionists recommend eating as few trans fats as possible in your diet. In fact, in some public grade schools in California, trans fats are banned from the campus and are not sold or provided by cafeterias.
|
|
|
|
Triglycerides and Health
|
Saturated and trans fats are harmful to health.
Saturated and trans fats lower “good” cholesterol and raise the “bad” cholesterol As of January 2006 trans fat content is required on the food label |
The effect of saturated and trans fats on the body is to lower the “good” cholesterol, HDL, and raise “bad” cholesterol, LDL, and since no studies that I know of have had any good findings for trans fats, their listing on food labels is now required by the FDA. Both saturated and trans fats are deemed to increase the risk of cardiovascular disease.
|
|
|
Fatty Acids and Health
|
Essential fatty acids
Two fatty acids cannot be synthesized in the body and must be obtained in the diet Linoleic and alpha-linolenic acid are essential (required in the diet) fatty acids. They are converted into important regulatory compounds in the body. |
There are two fatty acids that can’t be synthesized by the human body and are essential to health, so they must be obtained in the diet. They are linoleic acid and linolenic acid. Both are polyunsaturated fatty acids. They are called essential fatty acids because they are required in the diet to maintain good health. Both are converted into important regulatory compounds in the body.
|
|
|
Linoleic acid
|
Linoleic acid (omega-6 fatty acid)
Found in vegetable and nut oils Required for cell membrane structure Converted by the body to arachidonic acid which is involved in blood clotting and blood pressure Prostaglandins Thromboxanes Leukotrienes Lack of it in the diet leads to poor growth and scaly skin lesions |
Linoleic acid is the essential fatty acid that is the primary member of the omega-6 fatty acid family and is found in vegetable and nut oils. It can be converted to arachidonic acid by the body, and arachidonic acid is the precursor for some important compounds involved in blood clotting and blood pressure, the prostaglandins, thromboxanes, and leukotrienes.
|
|
|
Alpha-linolenic acid (omega-3 fatty acid)
|
Found in vegetables, fish, and fish oils
Converted to EPA and DHA which are important regulators of inflammation, blood clotting, and blood pressure, which improve vascular function May lower the risk of heart disease by reducing inflammation, plasma triglycerides, cardiac arrhythmias, blood pressure, and the development of blood clots |
The main member of the omega-3 fatty acid family is the essential fatty acid, linolenic acid. It is found in vegetables, fish, and fish oils. In the body, it can be converted to eicosapentaenoic acid, EPA, and docosahexaenoic acid, DHA, which are important regulators of inflammation, blood clotting, and blood pressure. They are both anti-inflammatory and decrease the risk of heart disease. The thromboxanes made from the omega-3 family are slippery and resist forming clots, which may be one reason for their positive effect on cardiovascular disease.
|
|
|
Phospholipids
|
Are composed of
Glycerol backbone Two fatty acids Phosphate Are amphipathic, which means they have both hydrophilic and hydrophobic properties Are manufactured in our bodies so they are not required in our diet Important component of cell membranes |
Molecules that have both hydrophilic and hydrophobic properties are amphipathic, and phospholipids qualify because they have a polar phosphate head that is hydrophilic and two lipid tails that are hydrophobic. They are not required in our diet because we derive them from triglycerides. The body removes one of the three fatty acids on a triglyceride and replaces it with a phosphate to create a phospholipid. They are extremely important because they are the main components of cell membranes.
|
|
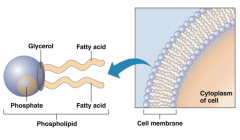
The drawing on this slide shows the structure of a phospholipid on the left and a cell membrane on the right. The hydrophilic polar phosphate head is attached to glycerol as are the two hydrophobic fatty acid tails. The phosphate head will face water and the lipid tails will turn away from it. This can be seen in the picture of the cell membrane. The membrane is composed of a phospholipid bilayer, i.e., there are two sets of phospholipids that together form the membrane. The environment outside of the cell is water-based and so is the cytoplasm inside of the cell. Thus, the phosphate heads of each layer face the water outside and inside of the cell while the hydrophobic lipid tails from each layer are sandwiched in between them facing each other. This creates a barrier, which helps establish order in the cell.
|
.
|
|
|
|
Sterols
|
lipids containing multiple 4 fused rings of carbon atoms
Are essential components of cell membranes and many hormones Are manufactured in our bodies and therefore are not essential components of our diet Cholesterol is the major sterol found in the body Is the precursor of the sex hormones (testosterone, estrogen, and progesterone), bile salts, vitamin D made in the skin in response to sunlight, and the corticosteroid hormones (cortisol) |
Sterols are sometimes referred to as steroids. The words mean the same thing. The major sterol and precursor of other sterols in the body is cholesterol. It is composed of four fused rings of carbon atoms surrounded by hydrogen atoms except for one hydroxyl group at the bottom of the first ring. Cholesterol has a bad reputation because of its association with heart disease, but it is a very important molecule that is routinely synthesized by the liver. It is a membrane component that functions to stabilize the membrane. In addition, it is the precursor of the sex hormones, the corticosteroid hormones, the bile salts, and vitamin D that is made in your skin in response to sunlight.
|
|
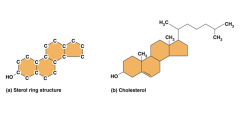
Here is a picture of the sterol ring on the left and cholesterol on the right. Both of them have a hydroxyl group at the bottom, which is the only part of the molecules that is hydrophilic. Otherwise, they are both made out of carbon and hydrogen atoms, which make them extremely hydrophobic. When cholesterol is inserted into a cell membrane, the –OH group sits next to the hydrophilic phosphate heads of the bilayer while the rest of the rings are positioned next to the fatty acid tails.
|
.
|
.
|
|
|
The role of fat: Energy
|
Fat is very energy dense, containing 9 kcal/gram
Much of the energy used during rest comes from fat Fat is used for energy during exercise, especially after glycogen is depleted Fat is also used for energy storage |
Fat has over twice as much energy as either carbohydrate or protein. It contains 9 kcal per gram, while carbohydrate and protein each contain 4 kcal per gram. Fat is used for energy, but the body employs it mostly during rest or low-level activity. If a person exercises for a long period of time, blood sugar will drop and glycogen will be depleted, after which the body will start to burn fat and protein. Since fat is so energy dense, it is a perfect energy storage molecule, and we store it as adipose tissue on our bodies.
|
|
|
Fat-soluble vitamins
|
Vitamins A, D, E, and K are soluble in fat; fat is required for their transport
|
.
|
|
|
Fat is essential to many body functions
|
Cell membrane structure
Nerve cell transmissions Protection of internal organs Insulation to retain body heat |
If you ever take fat-soluble vitamins, be sure to take them with a meal that has fat in it. Otherwise, you won’t be able to absorb the vitamins. The fat-soluble vitamins are A, D, E, and K, and fat is required for their absorption and transport. Fat is essential for many body functions including cell membrane structure, nerve cell transmission, cushioning and protection of internal organs, and as insulation to retain body heat.
|
|
|
The role of fat
|
Fat provides flavor and texture to foods
Fat contributes to making us feel satiated because Fats are more energy dense than carbohydrates or protein Fats take longer to digest |
In food, fat provides flavor and texture. It also contributes to our sense of feeling full possibly because of its high energy density and because it takes longer to digest than carbohydrate or protein. However, it is so energy dense that you can eat a lot more of it without feeling full. For example, a medium apple weighs about 117 grams and contains about 70 kcal, but you can get 70 kcal from only two pats of butter, and you would hardly feel full. So, fat probably provides a greater sense of satiety when it’s combined with protein and carbohydrate.
|
|
|
Digestion of Fats
|
Fats are not digested and absorbed easily because they are insoluble in water
Very little digestion of fats occurs in the watery environments of the mouth although salivary lipase is secreted Minimal digestion occurs in the stomach from gastric lipase Digestion and absorption of fats occurs primarily in the small intestine |
Fats pose a bit of a problem for digestion because our bodies are largely made out of water, and the enzymes that digest fat are water-soluble as well. But fat is not water-soluble, and therefore, very little fat digestion occurs in the watery environments of the mouth and stomach. There is an enzyme called salivary lipase that is secreted in the mouth, and a gastric lipase is secreted in the stomach, but the amount of digestion that takes place is minimal. In the small intestine, due to the presence of bile and pancreatic lipases, the pace and amount of fat digestion increases and most fat absorption occurs there.
|
|
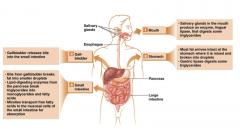
This sketch shows the details of fat digestion. When you begin to chew a food containing fat, the salivary glands produce lingual lipase that digests some triglycerides. The food is swallowed and arrives at the stomach where it is mixed and broken into fat droplets. The chief cells of the stomach secrete gastric lipase, and it digests some of the triglycerides. However, the amount of fat digested up to this point is still very small. The gallbladder releases bile into the small intestine, and chyme from the stomach, which contains fat molecules, passes slowly into the small intestine. The role of bile is not to digest fat in the sense of hydrolysis, but rather, to emulsify it. Emulsification is the breaking of large fat droplets into smaller ones that have more surface area and can be attacked by hydrolyzing pancreatic lipases. They cleave the fat into free fatty acids and monoglycerides. The latter have one fatty acid attached to glycerol and are the largest fat molecule that
|
.
|
.
|
|
|
Digestion of fats: Small intestine
|
As fat enters the small intestine
Bile is secreted from the gall bladder into the small intestine Bile is produced by the liver and stored in the gall bladder Bile disperses fat into smaller fat droplets Pancreatic enzymes break triglycerides into two separate fatty acids and a monoglyceride Fat enters the mucosal cell as a spherical micelle (fatty acids, monoglycerides, phospholipids, and sterols) |
The information on this slide reiterates what was said on the last slide, e.g., bile that is synthesized by the liver and stored in the gallbladder is secreted from the gallbladder into the small intestine. It emulsifies large fat droplets into smaller droplets that can be hydrolyzed by pancreatic lipases into free fatty acids and monoglycerides. These are escorted by spherical micelles into the mucosal cells for absorption.
|
|
|
Digestion of fats: the intestinal mucosal cell
|
In the intestinal mucosal cell
Fatty acids are reattached to the monoglyceride to reform triglycerides A small amount of protein is added to the lipids forming a chylomicron Chylomicron: a lipoprotein produced by cells lining the small intestine Composed of triglycerides surrounded by phospholipids and proteins Protein part is soluble in water |
After absorption takes place in the enterocytes, free fatty acids are reattached to monoglycerides to reform triglycerides. A small amount of protein is added to the lipids forming a chylomicron. The protein is added because it is water-soluble and will help the fats travel in the body’s water-based environment. Chylomicrons are formed following the absorption of fat from a meal, and they are lipoproteins. The main role of chylomicrons is to deliver dietary lipid to muscle cells, adipose cells, and tissues other than the liver. Fat-soluble vitamins are packaged in the chylomicrons along with free cholesterol, cholesterol esters, phospholipids, and triglycerides. The latter are present in the largest amount. Chylomicrons enter the lymph system because they contain too much hydrophobic lipid to enter the bloodstream.
|
|
|
Digestion of fats: the intestinal mucosal cell continued
|
Continued from side 3: As triglycerides and other lipids are removed from chylomicrons, they become somewhat less enriched in fats and are able to enter the bloodstream and travel to the liver where they are repackaged.
|
.
|
|
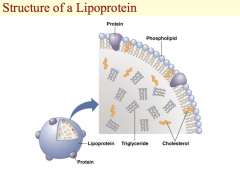
This is an illustration of a generic lipoprotein. The water-soluble proteins are on the outside of the particle along with the phosphate portion of phospholipids and the hydroxyl groups of cholesterol. The fat-soluble items are inside the lipoprotein.
|
.
|
.
|
|
|
Digestion of fats: Chylomicrons
|
Chylomicrons are the transport vehicle that removes absorbed fats from the small intestine.
Travel through the lymphatic system Are transferred to the bloodstream Short- and medium-chain fatty acids are absorbed more quickly since they are not arranged into chylomicrons. |
Chylomicrons first travel through the lymph system before entering the bloodstream because they are so enriched in hydrophobic fat molecules. They enter the bloodstream at a slow rate to prevent large-scale changes in the lipid composition of peripheral blood. In fact, entry of chylomicrons into the blood from the lymph can continue for up to 14 hours after consumption of a large meal rich in fat. As the chylomicrons travel through the bloodstream, more triglycerides are removed, and they become a chylomicron remnant, which is relatively less rich in triglycerides and relatively more rich in cholesterol. These are taken up by the liver. Short- and medium-chain fatty acids are absorbed and transported directly into the blood by the protein albumin. Thus, their transit time is quicker.
|
|
|
Digestion of fats: Chylomicrons continued
|
Once the chylomicron gets to a cell in the body, the triglycerides in the chylomicrons must be disassembled by lipoprotein lipase into two fatty acids and a monoglyceride before they can pass through the cell membrane
After entering the cell, the two fatty acids and monoglyceride reform a triglyceride The triglyceride can be Used immediately for energy Used to make lipid-containing compounds Stored in liver and muscle cells |
Fats are removed from chylomicrons by an enzyme called lipoprotein lipase. It disassembles triglycerides into two free fatty acids and a monoglyceride, which is very similar to what happened in the small intestine prior to absorption by the mucosal cells. Triglycerides are too large to pass through cell membranes, so the same process has to occur before they can enter a muscle or adipose cell. After they enter a cell, the two fatty acids are attached to the monoglyceride reforming a triglyceride, which can be used for energy or for making lipid-containing compounds. They can also be stored in the liver and muscle cells.
|
|
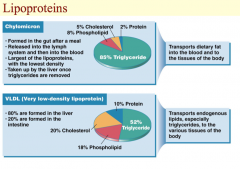
Pictured on this slide are two types of lipoproteins, a chylomicron and a very low- density lipoprotein (VLDL). Chylomicrons are formed in the enterocytes after a meal and released into the lymph as we discussed. They are the largest of the lipoproteins and have the lowest density due to high fat content but low protein content. Protein is more dense than fat. After they enter the blood, they are taken up by the liver. Chylomicrons contain approximately 85% triglyceride, 8% phospholipid, 5% cholesterol, and 2% protein. In contrast, 80% of VLDL is formed by the liver and 20% by intestinal cells. They contain about 52% triglyceride, 18% phospholipid, 20% cholesterol, and 10% protein. VLDL transports endogenous lipids, especially triglycerides, to the various tissues of the body.
|
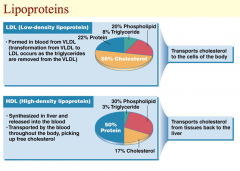
The figure on this slide features low-density lipoprotein (LDL) and high-density protein (HDL). As triglycerides are removed from VLDL in the blood, VLDL becomes LDL. LDL is enriched in cholesterol, and its job is to transport cholesterol to the cells of the body. High levels of LDL and cholesterol are associated with heart disease, and this is the reason that LDL has been called the “bad” cholesterol. LDL contains about 50% cholesterol, 22% protein, 20% phospholipid, and 8% triglyceride. In comparison, HDL is referred to as the “good” cholesterol because it picks up and transports cholesterol from tissues back to the liver for possible excretion in the bile. The benefit to the cardiovascular system is that by reducing the amount of deposited cholesterol in the vascular endothelium, HDL reduces the risk of fatty acid plaque formation, and therefore, of cardiovascular disease. HDL contains approximately 50% protein, 30% phospholipid, 17% cholesterol, and 3% triglyceride.
|
|
|
|
How Much Fat?
|
The Acceptable Macronutrient Distribution Range (AMDR) for fat
20–35% of calories should be from fat Athletes and highly active people may need more energy from carbohydrates and can reduce their fat intake to 20–25% of total calories. |
The type of fat consumed is important
Saturated fat should be no more than 7% of total calories Trans fatty acids should be reduced to the absolute minimum Most fat in our diets should be from monounsaturated fats (e.g., olive oil) and polyunsaturated fats (e.g., plant oils) |
|
|
Food Sources of Fat
|
Visible fats
Fats we knowingly add to foods Butter, cream, mayonnaise, dressings Invisible fats Fats hidden in foods Naturally occurring or added during processing |
Fats that we knowingly add to foods are called visible fats. Examples are butter, cream, mayonnaise, and salad or other types of dressings. Invisible fats are those that are hidden in foods. They could be naturally occurring or added during processing. An example of the latter is trans fats, which are found in many baked goods. It’s a good idea to look at the Nutrition Facts Panel when purchasing packaged foods.
|
|
|
Beneficial Fats
|
Increased consumption of “good” fats is advised
Increase consumption of omega-3 fatty acids Flaxseed oil, fish, and walnuts Avoid consumption of contaminated fish Mercury, PCB’s levels can be high some fish |
Increasing consumption of “good” fats is advised. Omega-3 fatty acids have become increasingly popular in that regard. Researchers studying native people who live near the Arctic wondered why they didn’t have cardiovascular disease since their diet consisted primarily of fatty fish and other meats with no appreciable amount of fruits or vegetables. It was found that fatty fish like salmon contain high amounts of omega-3 fatty acids, and that they are anti-inflammatory. Many heart specialists believe that cardiovascular disease is associated with low-grade inflammation, and omega-3 fats may help reduce inflammation, which is one reason why they are beneficial. In addition, it’s known that omega-3 fatty acids contribute to the production of thromboxanes that are more slippery and less likely to clot, which makes a stroke or heart attack less likely.
|
|
|
Beneficial Fats continued
|
Continued from side 3: Omega-3 fats are found in salmon and sardines, flaxseed oil, and walnuts. There are also many foods that have been modified to contain omega-3 fats such as eggs and soy milk. Fish that have omega-3 fatty acids may pose a problem due to contamination by mercury. In order to obtain omega-3 fats by eating fish, you would have to eat it on a regular basis, and some people are worried about heavy metal contamination, so they try to get the omega-3 fats from a different food. Omega-3 fats are also heavily marketed as supplements.
|
.
|
|
|
Fat Replacers
|
Many fat replacers have been introduced
Olestra and other fat replacers have been introduced to help people reduce caloric intake May cause gastrointestinal distress |
Fat replacers were introduced primarily to help people lose weight, but many of them such as Olestra, cause gastrointestinal distress, which is a euphemism for some nasty symptoms. However, studies indicate that they don’t result in much weight loss, so most nutritionists don’t recommend them.
|
|
|
Health Problems from Fat
|
Cardiovascular disease
Dysfunction of the heart or blood vessels Can result in heart attack or stroke The type of fat in our diet can contribute to or help protect against cardiovascular disease. |
A high fat diet is recognized as contributing to cardiovascular disease, which can result in a heart attack or stroke. However, the type of fat consumed in the diet is important. The current thinking is that we should lower saturated fat consumption, avoid trans fats altogether, increase our intake of omega-3 fats, continue to consume omega-6 fatty acids, which are found in most vegetables and vegetable oils, and use oils such as olive oil and canola oil, which are high in monounsaturated fatty acids. The reason for increasing olive and canola oil use is that nutritionists feel that the balance between omega-3 and omega-6 fatty acids is skewed in the American diet.
|
|
|
Health Problems from Fat continued
|
continued from side 3: When we introduced corn oil and regular vegetable oils into the diet, we upset the balance. Both of these oils are high in omega-6, but very low in omega-3 fats. Experts believe that the ratio of omega-6 to omega- 3 in the American diet used to be about 1 to 1, but following the advent of corn and vegetable oils, which have been widely used for cooking all of our foods, the ratio changed to about 25 to 1 on average. Omega-6 fatty acids are necessary, but they are pro-inflammatory. This is not bad because you need to be able to mount an inflammatory response, but having too much of them and not enough of the omega-3 anti-inflammatory fatty acids is unbalanced. Thus, if you try to consume more omega-3 fats and use olive and canola oils for everyday purposes instead of corn and generic vegetable oils, the balance between omega-6 and omega-3 could be restored.
|
.
|
|
|
Cardiovascular Disease
|
Risk factors for cardiovascular disease include
Being overweight Physical inactivity Smoking High blood pressure Diabetes High blood cholesterol |
Risk factors for cardiovascular disease include being overweight, lack of physical activity, smoking, high blood pressure, diabetes, and high blood cholesterol.
|
|
|
Cardiovascular Disease: Blood Lipids
|
Blood lipids include
Chylomicrons VLDLs—very low-density lipoproteins LDLs—low-density lipoproteins “bad cholesterol” HDLs—high-density lipoproteins “good cholesterol” |
We’ve gone over the blood lipids, so this slide is somewhat of a review, but chylomicrons are the lipoproteins that transport lipids into the lymph system immediately after a meal. They enter the blood and become a chylomicron remnant that is absorbed by the liver, which repackages them into VLDL. The latter enters the blood and as fats are removed becomes an LDL whose primary job is to deliver cholesterol to the cells. Since high cholesterol is an indicator of cardiovascular disease, LDL has been labeled the “bad” cholesterol. The liver also makes HDL whose function is to remove cholesterol from the cardiovascular system, so it has been labeled the “good” cholesterol.
|
|
|
Cardiovascular Disease: Diets high in sat fats
|
Diets high in saturated fats
Decrease the removal of LDLs from the blood Contribute to the formation of plaques that can block arteries Increase triglyceride levels (chylomicrons and VLDLs) |
When researchers were studying men who had experienced heart attacks in the 1950s, they found that almost all of them had high cholesterol, so cholesterol became a helpful indicator of the potential for cardiovascular disease. However, as more research was conducted, they found out that we have a feedback system in which higher consumption of cholesterol in the diet results in lower production of cholesterol by the liver. So, unless we regularly over eat foods that are high in cholesterol, most of us won’t have a problem with it. Further research indicated that high cholesterol levels may be perpetuated by eating a diet high in saturated fat, so now many professionals feel that saturated fat is more of a problem than cholesterol. Saturated fats appear to decrease the removal of LDLs from the blood contributing to plaque formation resulting in arterial blockage. Consuming too much saturated fat has also been linked to increased triglyceride levels, another indicator of heart disease.
|
|
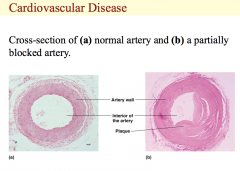
Cross-section of (a) normal artery and (b) a partially blocked artery.
This illustration shows a normal artery on the left. The opening in the artery is wide and clear allowing normal blood flow. On the right is a partially blocked artery that displays plaque build-up and a narrowing of the arterial passageway that restricts blood flow and could result in a heart attack. |
.
|
.
|
|
|
Cardiovascular disease: Trans fatty acids
|
Trans fatty acids
Can raise blood cholesterol levels more than saturated fat Are abundant in hydrogenated vegetable oils (margarine, vegetable oil spreads) Should be reduced to the absolute minimum |
Studies have shown that trans fats can raise blood cholesterol levels even more than saturated fats. We know that they are the product of hydrogenation and do not occur naturally, and research results have been overwhelmingly negative, so it is recommended that they be avoided.
|
|
|
How can fat intake protect against heart disease?
|
Diets high in omega-3 fatty acids (along with moderate exercise) can increase HDL “good” cholesterol levels.
|
It is recommended that omega-3 fatty acid intake be increased along with moderate exercise. Both are associated with increased HDL. This will help reduce the risk of cardiovascular disease.
|

