![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
123 Cards in this Set
- Front
- Back
|
What is the initial step in formation of the nervous system? |
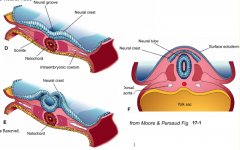
- Neurulation (Primary and Secondary)
- Converts flat Neural Plate into a Neural Tube (precursor of CNS) |
|
|
What are the precursors of the nervous system?
|
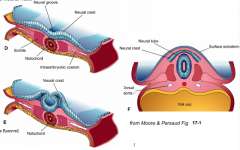
Three areas of ectoderm:
- Neural Plate - Neural Crest - Ectodermal Placodes |
|
|
What are the phases of primary neurulation?
|
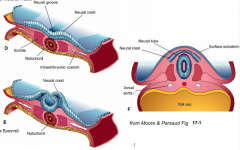
1. Neural Plate Induction and Formation
2. Shaping of the Neural Plate 3. Formation of Neural Folds 4. Neural Fold Fusion |
|
|
What happens during the first phase of primary neurulation?
|
Neural Plate Induction and Formation
** Primitive node signals induces ectoderm --> neural plate - Down-regulates expression of BMP-4 - Leads to FGF expression in ectoderm - Causes cell proliferation and thickening --> Neural Plate / Neuroepithelium |
|
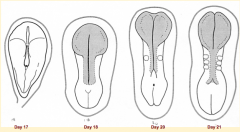
What happens during the second phase of primary neurulation (after neural plate induction and formation)?
|
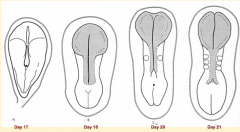
Shaping of Neural Plate:
- Expands cranially --> Brain Precursor - Caudal portion narrows and lengthens --> Spinal Cord Precursor (via Convergent Extension) |
|
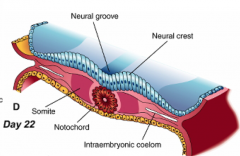
What happens during the third phase of primary neurulation (after shaping of neural plate)?
|
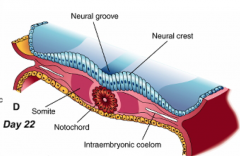
Formation of Neural Folds:
** Neural Plate folds dorsally --> Neural Tube - Neuroepithelial cells directly above notochord change shape to become a "Median Hinge Point" / pivot - Midline furrow = neural groove - Neural folds contain neuroepithelium and surface ectoderm |
|
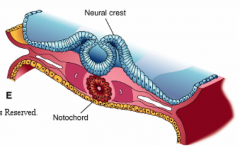
What happens during the fourth phase of primary neurulation (after formation of neural folds)?
|
Neural Fold Fusion
- Neural folds appose and adhere - Form two layers of epithelia: outer layer of surface ectoderm and inner layer that forms roof plate of neural tube - Begins fusion in cervical and occipital regions --> continues cranially and caudally (temporary opening to amniotic fluid = neuropores) - Ventral flexure forms = midbrain (cranially = forebrain; caudally = hindbrain) - Neuroepithelial cells --> epithelial mesenchymal transformation --> neural crest cells (cranially before fusion, caudally after fusion) |
|
|
During what stage does the neural tube undergo a flexure? Relationship of structures to flexure?
|
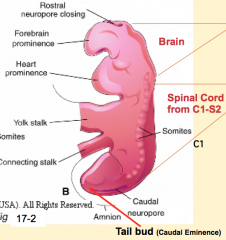
- Phase 4 of primary neurulation (neural fold fusion)
- Forebrain = cranial to flexure - Midbrain = at flexure - Hindbrain = caudal to flexure |
|
|
What cell transformation happens at the end of phase 4 of primary neurulation? Timing?
|
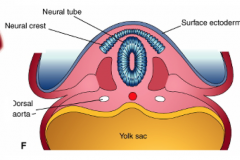
- Neuroepithelial cells at interface of neuroepithelium and surface ectoderm
- Epithelial mesenchymal transformationg - Forms neural crest cells - Cranially - occurs prior to fusion of neural folds - Caudally - occurs after fusion of neural folds |
|
|
When do the neuropores close?
|
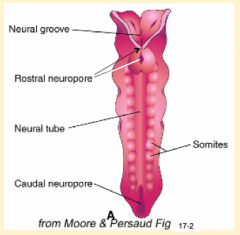
- Cranial/rostral neuropore closes first (days 24-27)
- Caudal neuropore closes after (days 26-30) |
|
|
What is the CNS derived from?
|
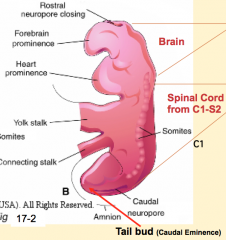
- Brain and spinal cord from C1 - S2: Neural Tube (primary neurulation)
- Spinal cord from S3 - lower: Tail Bud / Neural Cord (secondary neurulation) |
|
|
What is the function of secondary neurulation?
|
Forms spinal cord from S3 to Coccygeal levels
|
|
|
What are the phases of secondary neurulation?
|
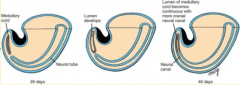
1. Neural (medullary) cord formation
2. Lumen formation 3. Regression of the tail bud (caudal eminence) |
|
|
What happens during the first phase of secondary neurulation?
|
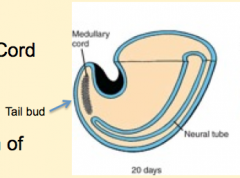
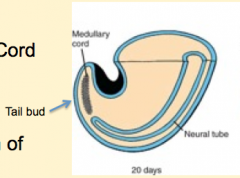
Neural (Medullary) Cord Formation:
- Begin with mass of undifferentiated mesenchyme in tailbud - Forms a solid cord of cells called the Neural Cord |
|
What happens during the second phase of secondary neurulation (after formation of the neural cord)?
|
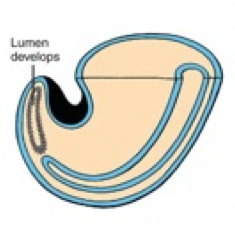
Lumen Formation
|
|
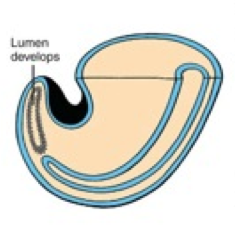
What happens during the third phase of secondary neurulation (after lumen formation)?
|
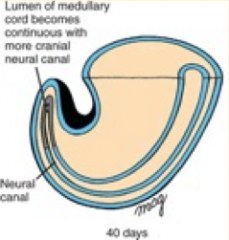
Regression of Tail Bud (Caudal Eminence):
- Lumen becomes continuous w/ central canal of spinal cord formed from neural tube - Tailbud regresses |
|
|
How common are congenital defects of the nervous system?
|
- 75% of fetal and 40% of infant deaths are attributed to congenital malformations from defects of the nervous system
- 10% of all malformations |
|
|
What are the types of errors of neurulation / neural tube defects?
|
- Errors of Secondary Neurulation: closed neural tube defects
- Errors of Primary Neurulation: open neural tube defects / dysraphic errors |
|
|
What characterizes secondary neurulation defects?
|
Closed neural tube defects:
- Abnormal formation of caudal part of spinal cord from tail bud (caudal eminence) - Caudal location (at ends of neural tube); usually skin over lesion is intact |
|
|
What are the more common types of secondary neurulation errors?
|
- Lipomyelomeningocele
- Lipomeningocele - Tethered cord |
|
|
What characterizes primary neurulation defects?
|
Open neural tube defects / dysraphic errors:
- Faulty Neural Tube closure - Dorsal location / along midline - Affects the skeletal and neural tissue |
|
|
What are the more common types of primary neurulation errors?
|
- Spina Bifida
- (Mero)anencephaly - Encephalocele |
|
|
What can protect the embryo somewhat from developing neural tube defects?
|
Maternal folic acid supplements (400 µg / day)
|
|
|
What are the examples of defects affecting the neural tube?
|
Affecting skull:
- (Mero)anencephaly - Encephalocele - Cranial Meningocele - Cranium Bifidum Affecting spinal cord: - Myeloschisis - Meningmyelocele - Meningocele - Spina Bifid Occulta |
|
|
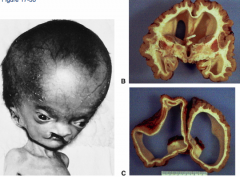
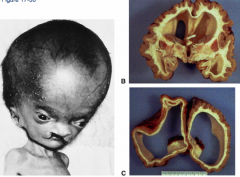
What defect is characterized by the absence of the calvaria and a rudimentary mass of forebrain and midbrain tissue exposed dorsally?
|
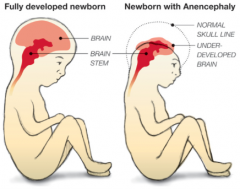
(Mero)Anencephaly
|
|
|
What are the characteristics of (Mero)Anencephaly?
|
* Absence of calvaria
* Rudimentary mass of forebrain and midbrain tissue exposed dorsally * Presence of brainstem - Normal eyes (some forebrain development) - Cleft palate - Atrophy of fetal adrenal cortex - Excess amniotic fluid due to inability to swallow |
|
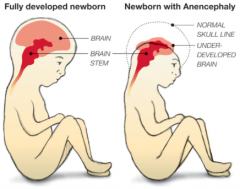
How common is (Mero)Anencephaly? What is it associated with?
|
- 1/1000 live births
- More prevalent in females (3:1) - Autosomal link - Increased incidence in embryos conceived in January and infants of Celtic descent |
|
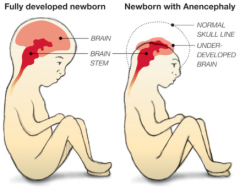
How do you diagnose (Mero)Anencephaly?
|
- Ultrasound
- Increased Alpha Fetoprotein levels in maternal serum |
|
|
What defect is characterized by a skull defect called bifid cranium (usually in the occipital bone) where portions of the brain and its meningial coverings herniate through defect?
|
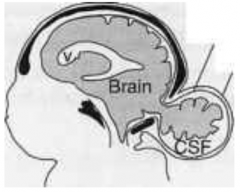
Encephalocele (or Meningoencephalocele)
|
|
|
What are the characteristics of Encephalocele (or Meningoencephalocele)?
|
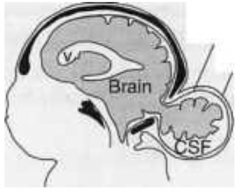
* Skull defect (bifid cranium), usually in occipital bone
* Portions of brain and meninges herniate through defect * Enclosed by skin - Hydrocephalus - Polydactyly - Polycystic kidneys - Quadriplegia and incontinence common |
|
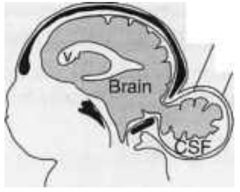
How common is Encephalocele (or Meningoencephalocele)?
|
1/2000 live births
|
|
|
What defect is characterized by a small defect in the skull but with no brain tissue herniation (only meninges herniate)?
|
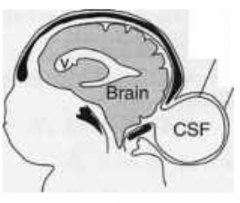
Cranial Meningocele
|
|
|
What are the characteristics of Cranial Meningocele?
|
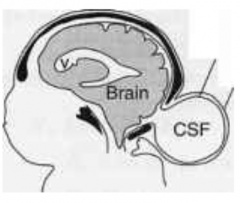
- Small defect in skull
- No brain tissue herniation - Meninges pass through defect - Forms a sac beneath skin filled w/ CSF |
|
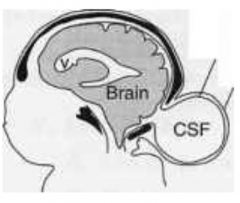
How common is Cranial Meningocele?
|
1/2000 live births
|
|
|
What is the term for an opening between the skull bones (usually occipital bone but sometimes frontal bone)?
|
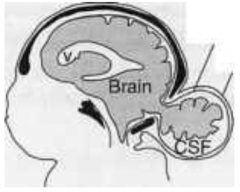
Cranium Bifidium (interchangeably used w/ encephalocele which refers to herniation of brain/meninges through opening)
|
|
|
What term is used to describe neural tube defects in the spinal cord?
|
Spina Bifida
|
|
|
What defect is characterized by a defect in the formation of the vertebral arch combined with a neural plate that is open dorsally, with no cystic swelling?
|
Myeloschisis
|
|
|
What are the characteristics of Myeloschisis?
|
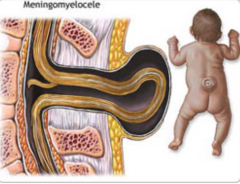
- Spina Bifida Aperta (Open)
- Very rare - Defect in formation of vertebral arch * Neural plate open dorsally to surface - No associated cystic swelling - One or more spinal cord levels may be involved |
|
|
What defect is characterized by a defect in the formation of the vertebral arch combined w/ a cystic swelling (containing spinal cord and meninges) that may be covered with skin or a membrane?
|
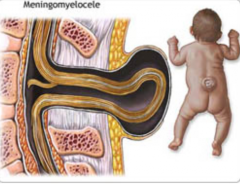
Meningomyelocele
|
|
|
What are the characteristics of a Meningomyelocele?
|
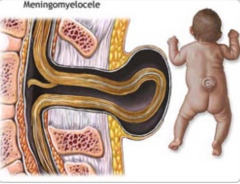
- Defect in formation of vertebral arch
* Cystic swelling containing spinal cord and meninges - May be covered with skin or a membrane * Usually at lumbosacral level - Hydrocephalus and club feet often - Urinary incontinence and paraplegia often |
|
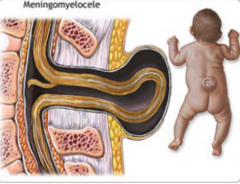
How common is Meningomyelocele?
|
- 1-5/1000 live births
- 90% of spina bifida cystica cases |
|
|
What defect is characterized by a defect in the vertebral arch formation and a herniated meningeal sac filled w/ CSF?
|
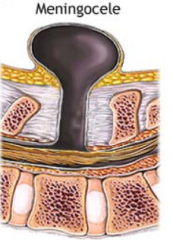
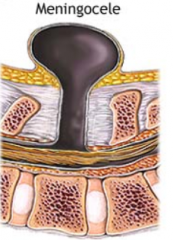
Meningocele
|
|
|
What are the characteristics of Meningocele?
|
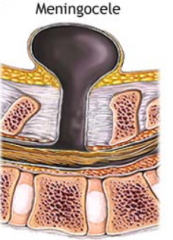
- Defect in vertebral arch
- Herniated meningeal sac filled w/ CSF - Spinal cord anomalies may be associated - Arises in late embryonic or early fetal period |
|
How common is Meningocele?
|
- 1/1000 live births
- 10% of spina bifida cases |
|
|
What is Spina Bifida Cystica?
|
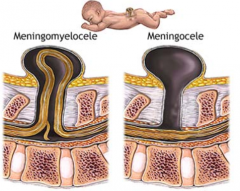
- Meningocele
- Meningomyelocele - Both classified as this |
|
|
What defect is characterized by a defect in formation of vertebral spinous process (limited to 1-2 vertebrae) and is usually asymptomatic?
|
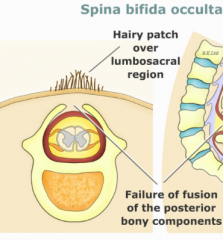
Spina Bifida Occulta
|
|
|
What characterizes Spina Bifida Occulta?
|
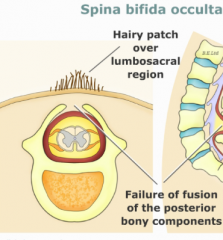
- Defect in formation of vertebral spinous process
- Limited to 1-2 vertebral levels - Usually asymptomatic - May be associated with a skin dimple, fatty cyst, hair tuft/dimple (diastematomyelia) |
|
|
What is Diastematomyelia?
|
- Bone spur in spinal canal that can tether the spinal cord and keep it from ascension in spinal canal
- Associated with hair tuft/dimple |
|
|
How do you diagnose neural tube defects?
|
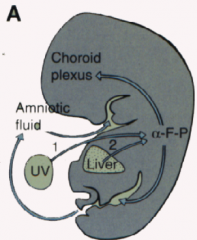
- Increase in alphafetoprotein levels in maternal serum
- Ultrasound confirmation |
|
|
What are the stages of formation of the spinal cord?
|
1. Proliferation of the neuroepithelial cells
2. Differentiation of the neuroepithelial cells 3. Neuron and Glial formation results in a trilaminar spinal cord wall 4. Patterning of the forming spinal cord 5. Positional changes of the spinal cord |
|
|
What happens during the first stage of spinal cord formation?
|
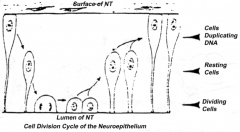
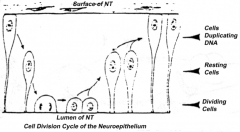
Proliferation of neuroepithelial cells:
- Cells proliferate - Nucleus moves within cytoplasm and in phase with cell cycle - Nuclei next to neural tube = S phase - Nuclei next to lumen are dividing = M phase |
|
|
How does the location of the nucleus in the neuroepithelium relate to the cell cycle? Where are these neuroepithelium?
|
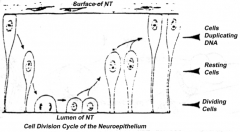
- Nuclei next to neural tube = S phase
- Nuclei next to lumen are dividing = M phase - Neuroepithelium of the spinal cord |
|
What happens during the second stage of spinal cord formation (after proliferation of neurepithelial cells)?
|
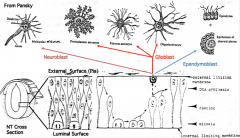
Differentiation of Neuroepithelial Cells:
- Into neurons or glial (supporting) cells - Neuroblasts (neuron forming cells) differentiate first (migrate toward surface of neural tube where they further differentiate) - Glioblasts (glia forming cells) differentiate after neuroblasts have formed (form astrocytes and oligodendrocytes = macroglia; also radial glia which serve as guideway for migration) - Ependymal cells form last and remain at same location (form epithelial cells that line ventricles and spinal canal) |
|
|
What are the cell types derived from the neuroepithelium of the neural tube?
|
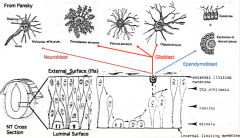
- Neurons
- Glial cells (astrocytes and oligodendroglia) - Ependymal cells |
|
|
What are the cell types derived from the blood cells (monocytes)?
|
Microglia cells (macrophages)
|
|
|
How do the neuroblasts know were to migrate to?
|
Radioglia (temporary glial cells that disappear after neurons reach their final location) - act as a guideway for migration
|
|
What happens during the third stage of spinal cord formation (after differentiation of neuroepithelial cells)?
|
Neuron and Glial Formation --> Trilaminar Spinal Cord Wall
- Original layer: Ventricular / Germinal Layer - continues to proliferate and remain adjacent to lumen; cells become ependymal cells that line central canal - Intermediate / Mantle Layer: contains neurons and glia, eventually remodeled into Gray matter - Marginal Layer: contains axons of neuroblasts; oligodendroglia also are here = White matter |
|
|
What layer of the spinal cord wall corresponds to the Gray Matter? White Matter?
|
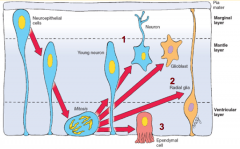
- Gray Matter = Intermediate or Mantle Layer
- White Matter = Marginal Layer |
|
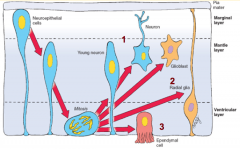
What happens during the fourth stage of spinal cord formation (after formation of trilaminar spinal cord wall)?
|
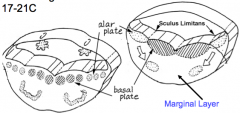
Patterning of the Forming Spinal Cord:
- Signaling molecules from surface ectoderm and notochord act on neuroblasts and glioblasts - Leads to dorsal-ventral pattern - No neurons or glia in wall of dorsal and ventral midline = Roof (dorsal) and Floor (ventral) Plates - Dorsal half of intermediate layer --> Alar Plates --> Sensory neurons (via dorsalizing signals) - Ventral half of intermediate layer --> Basal Plates --> Motor neurons (via ventralizing signals, Shh) - Lateral extensions of basal plates: (T1-L2) --> sympathetic motor column; (S2-S4) --> parasympathetic motor neurons |
|
|
What are the Alar and Basal Plates?
|
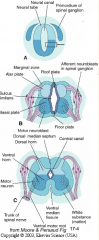
- Alar Plate = dorsal half of intermediate layer --> sensory neuron columns
- Basal Plate = ventral half of intermediate layer --> motor neuron columns |
|
|
What is the Sulcus Limitans?
|
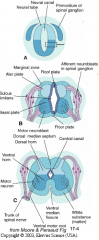
Groove running the entire length of the spinal cord forming a boundary line between sensory (dorsal) and motor (ventral) areas; remains present in mature spinal cord
|
|
|
What happens during the fifth stage of spinal cord formation (after patterning of forming spinal cord)?
|
Positional Changes of Spinal Cord:
- During fetal period, body of fetus, including vertebral column, grows faster (in length) than spinal cord - Caudal end of spinal cord is located at progressively higher vertebral levels - Filum Terminale attachment to sacrum serves as reference point for demonstrating spinal cord ascent |
|
|
What are the stages that take place during brain formation?
|
1. Affect of embryo folding on neural tube
2. Regionalization of forming brain 3. Formation of 3-layered neuroepithelium 4. Formation of ventricles 5. Formation of commissures 6. Formation of cerebellum and cerebral hemispheres |
|
|
What happens during the first stage of brain formation?
|
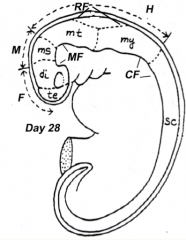
Embryo Folding
- Ventral flexure: Mesencephalic / Midbrain Flexure - Second ventral flexure: Cervical Flexure - junction of brain and spinal cord - Rapid growth and lengthening of brain after neural tube closes - Third flexure: Rhombencephalic (Pontine) Flexure in area of hindbrain (dorsal bending) - Neural tube bends dorsally causing the brain to fold back on itself |
|
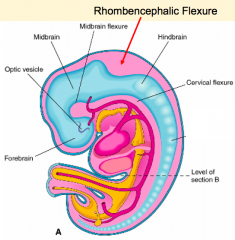
What happens during the second stage of brain formation (after folding of neural tube / rhombencephalic flexure)?
|
Regionalization of the Forming Brain
- Brain regions named in reference to Mesencephalic Flexure: Prosencephalon (cranial), Mesencephalon (at flexure), and Rombencephalon (caudal) - Prosencephalon divided into Telencephalon (cranial - becomes cerebral hemispheres) and Diencephalon (caudal) - Rhombencephalon divided into Metencephalon (cranial) and Myelencephalon (caudal) |
|
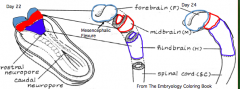
What does the Prosencephalon / Forebrain (cranial to mesencephalic flexure) divide into?
|
- Telencephalon (cranial) --> Cerebral Hemispheres
- Diencephalon (caudal) --> retains central position in forebrain |
|
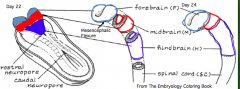
What does the Rhombencephalon / Hindbrain (caudal to mesencephalic flexure) divide into?
|
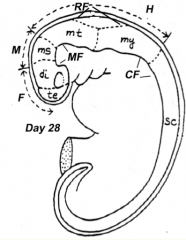
- Metencephalon (cranial)
- Myelencephalon (caudal) |
|
|
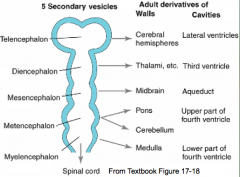
What are the five brain regions? What are they known as?
|
- Te = Telencephalon --> Cerebral Hemispheres
- Di = Diencephalon --> Thalamus, etc. - Ms = Mesencephalon --> Midbrain - Mt = Metencephalon --> Pons and Cerebellum - My = Myelencephalon --> Medulla - They are also known as Secondary Brain Vesicles (represented in adult brain) |
|
|
Where do the signals that organize the FOREBRAIN and MIDBRAIN originate?
|
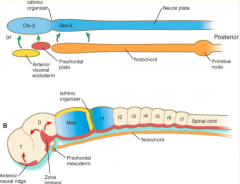
Prechordal plate (aka Prosencephalic Signaling Center)
Otx2, Lim1 (Also, the Isthmus Organizer sends signals to further organize the midbrain) |
|
|
Where do the signals that organize the HINDBRAIN originate?
|
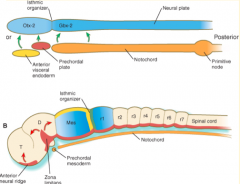
Notochord (aka Rhombencephalic Signaling Center)
Wnt3a, Fgf, RA |
|
|
What area is found between the Prosencephalic and Rhombencephalic Signaling Centers? Significance?
|
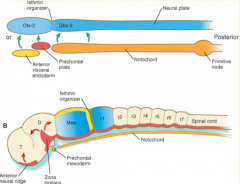
- Isthmus organizer at midbrain-hindbrain border
- Signals from this area lead to further organization of midbrain and mediate formation of the cerebellum |
|
|
Where do the signals that organize the CEREBELLUM originate?
|
Isthmus Organizer (also further organizes the midbrain)
|
|
|
How do the signals from the Prosencephalic Signaling Center (Prechordal Plate) affect the forebrain?
|
Forms subtle, transient subdivisions in forebrain called Prosomeres
|
|
|
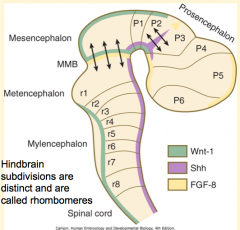
How do the signals from the Rhombencephalic Signaling Center (Notochord) affect the hindbrain? How?
|
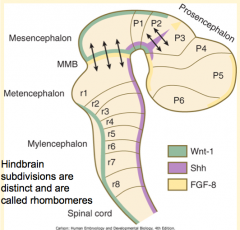
Subdivides the hindbrain into 7-8 temporary segments called Rhombomeres (by controlling expression of Hox genes)
|
|
|
What condition is characterized by the absent or incomplete regionalization of the prosencephalon (forebrain)?
|
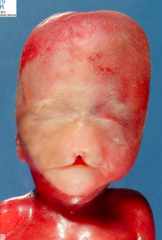
Holoprosencephaly
|
|
|
What are the characteristics of Holoprosencephaly?
|
- Regionalization of prosencephalon is absent or incomplete
- Midline CNS and facial malformations - Primarily affects forebrain - Corpus callosum, olfactory tract, and falx cerebri often absent - Single ventricle may be present - Seizures, delays in development, and midface anomalies |
|
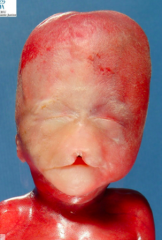
What causes the condition Holoprosencephaly?
|
- Chromosomal defect (50%)
- The rest are unknown - Maternal alcohol ingestion may contribute - Also mutations in SHH and cholesterol biosynthesis may be involved |
|
|
What is the growth of the brain in close association with?
|
Growth of the skull
|
|
|
What happens during the third stage of brain formation (after regionalization of the forming brain)?
|
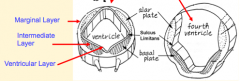
Development of 3-layered Neuroepithelium
- Brainstem: similar to spinal cord, forms Ventricular, Intermediate, and Marginal layers - Forebrain (telencephalon and diencephalon) only form an observable Ventricular layer |
|
|
What factors modify the neuroepithelium of the neural tube int he brain region?
|
- Cell migration
- Differential Growth - Differentiation - Apoptosis - All of these processes are under the direction of unique combinations of signaling molecules |
|
|
What are the general features of brainstem development once the trilaminar neuroepithelium forms?
|
- Alar and basal plates are present and located adjacent to lumen of neural tube
- CN Motor nuclei develop in Basal Plates (medial/anterior) - CN Sensory nuclei develop in Alar Plates (lateral/posterior) - Sulcus Limitans continues to cranial border of mesencephalon (defines interface between basal and alar plates) - Nerve tracts form in Marginal Layer - Some neurons migrate from alar plate to marginal layer and form specific brain nuclei |
|
|
What forms in the alar and basal plates of the brainstem?
|
- Alar Plates: CN Sensory Nuclei
- Basal Plates: CN Motor Nuclei |
|
|
What are the general features of forebrain development once the ventricular layer of neuroepithelium forms?
|
- Only Alar plate forms in forebrain
- Complexes of nuclei in forebrain are located next to the lumen (ventricles), analogous to intermediate layer - Telencephalon --> Basal Ganglia - Diencephalon --> Thalamus, Epithalamus, Hypothalamus - Fiber tracts develop in marginal layer |
|
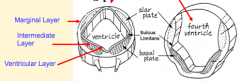
What happens during the fourth stage of brain formation (after formation of 3-layered neuroepithelium - brainstem, 1 layer - forebrain)?
|
Formation of the Ventricular System
- Lumen of neural tube becomes brain ventricular system - Ventricle forming in each brain region has its own unique morphology - Secretory epithelium (Choroid Plexus) forms in roof of most ventricles - source of CSF |
|
|
What are the origins of the ventricles?
|
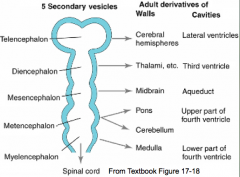
Cavities in these structures:
- Telencephalon --> Lateral Ventricles - Diencephalon --> Third Ventricle - Mesencephalon --> Cerebral Aqueduct - Metencephalon --> Upper part of 4th ventricle - Myelencephaon --> Lower part of 4th ventricle |
|
|
Where does the Choroid Plexus originate from?
|
Roof of most ventricles
|
|
|
What happens during the fifth stage of brain formation (after formation of ventricles)?
|
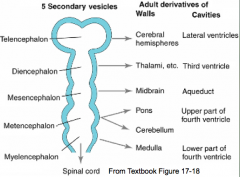
Formation of Commissures
- Fiber tracts called commissures connect cerebral hemispheres - Form the commissural plate (thickened area of prosencephalon at site of cranial neuropore closure) - Anterior commissure forms near cranial extent of telencephalon - Posterior commissure forms near caudal boundary - Corpus Callosum (largest commissure) forms caudal to anterior commissure |
|
What happens during the sixth stage of brain formation (after formation of the commissures)?
|
Formation of the Cerebellum and Cerebral Hemispheres
|
|
|
What is the cerebellum derived from and how does it form?
|
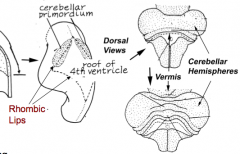
- Dorsal outgrowths of metencephalon, called the Rhombic Lips (part of Alar plate)
- Thombic lips fuse at midline to form Cerebellar Pate (fusion area = Vermis) - Hemisphere expands laterally on each side of vermis |
|
|
How many layers does the Cerebellar Cortex have? How do the layers form?
|
- 3 definitive layers: molecular layer (external), Purkinje layer (intermediate), and granular layer (internal)
- Neuroblasts in rhombic lips migrate to form External Granular Layer (Molecular Layer) beneath pia (external to marginal layer) - Other neuroblasts migrate into marginal (Purkinje) layer and form specialized cells called Purkinje cells - Cells of external granular layer move inward beneath Purkinje layer to form Inner Granular Layer (ninjas) - Granule cells use radial glial cells as migratory substratum to get to inner layer - Some neuroblasts remain in intermediate layer where they form Deep Cerebellar Nuclei (adjacent to 4th ventricle) |
|
|
What makes up the cerebral hemispheres?
|
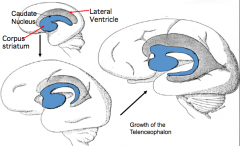
- Cerebral Cortex (external) - forms from thin roof and lateral walls
- Corpus Striatum or Basal Ganglia (internal adjacent to lateral ventricles) - forms from thick floor of each hemisphere |
|
|
What is the cerebrum derived from and how does it form?
|
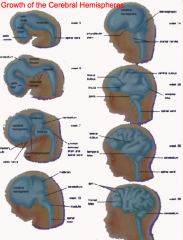
- Lateral outgrowths of Telencephalon
- Covers diencephalon - Continued growth leads to formation of gyri and sucli - Caudal end grows downward and then curves forward (to create temporal lobe) - Cranial end expands forward (to create frontal lobe) |
|
|
What does the Basal Ganglia form from?
|
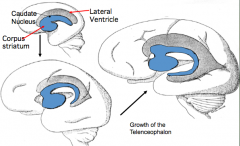
- Thick floor of each cerebral hemisphere, adjacent to the ventricle
- Aggregations of neuroblasts form an elevated Ganglion Eminence here |
|
|
What influences the shape of the ventricles and caudate nucleus?
|
Expansion of the cerebral hemispheres
|
|
|
How many layers does the cerebral cortex have? What are they?
|
6 layers (fewer in olfactory area and hypocampus) from deep (near ventricle) to superficial:
1. VZ = Ventricular zone 2. SVZ = Subventricular Zone 3. IZ = Intermediate Zone 4. SP = Subplate (from Preplate) 5. CP = Cortical Plate 6. MZ = Marginal Zone (from Preplate) |
|
|
How do the 6 layers of the cerebral cortex form?
|
1. Neurons from the Ventricular Zone (VZ) migrate outward forming the Preplate (PP)
2. Axons from the PP neurons extend inward forming an Intermediate Zone (IZ) (becomes white matter) 3. Second group of neurons migrate into PP forming the Cortical Plate (CP) splitting PP into Marginal Zone (MZ) and Subplate 4. MZ forms superficial to CP (MZ = layer 1 of cortex) 5. CP forms layers 5 & 6 (deepest layers of cortex) 6. Additional waves of neurons pass superficial to CP to form layers 4, 3, & 2 respectively 7. Cells in VZ become ependymal cells lining the ventricles ?? Confusing ?? |
|
|
Which layer of the cerebral cortex becomes the cortical white matter?
|

Intermediate Layer (IZ)
|
|
|
What happens to the cells in the Ventricular Layer after all of the neuroblasts and glioblasts are formed?
|
Becomes Ependymal cells
|
|
|
What percentage of the brain is formed at birth?
|
25% of adult volume
|
|
|
What kind of errors can occur involving the cerebral cortex?
|
- Errors of Neuron Proliferation
- Errors of Neuron Migration - Hydrocephalus |
|
|
Abnormal brain development involving the cerebral cortex often leads to what symptoms?
|
- Seizures
- Developmental Impairment - CSF Obstruction |
|
|
What are the errors of neuron proliferation involving the cerebral cortex?
|
- Megalencephaly
- Microencephaly |
|
|
What defect is characterized by increased neuron proliferation in the cerebral cortex? How does it affect the fetus?
|
- Megalencephaly
- Mental status may or may not be affected - May be familial |
|
|
What defect is characterized by decreased neuron proliferation in the cerebral cortex, leading to an undersized brain?
|
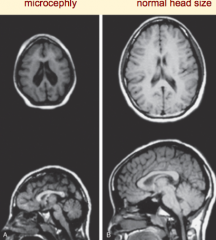
Microencephaly
|
|
|
What are the characteristics of Microencephaly?
|
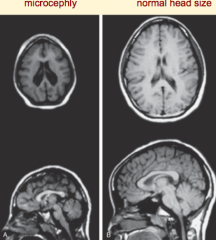
- Decreased neuron proliferation
- Undersized brain - Small cranial vault - Severe mental retardation - Associated w/ 150 syndromes (20% genetic, some teratogenic) |
|
|
What are the errors of neuron migration involving the cerebral cortex?
|
- Lissencephaly
- Microgyria - Macrogyria - Agenesis of the Corpus Callosum |
|
|
What defect is characterized by a cortical surface having macrogyria (larger gyri) or agyria (no gyri), causing a smooth brain?
|
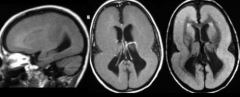
Lissencephaly = Smooth Brain
|
|
|
What characterizes Lissencephaly?
|
- Cortical surface w/ macrogyria (larger gyri / pacygria) or agyria (no gyri)
- Accompanies by Heterotopia (neurons in aberrant positions) - Decreased lamination of cortex - Poor responsiveness and feeding - Seizures - Decreased muscle tone - Microcephaly - Severe developmental delay |
|
|
What defect is characterized by the presence of excessive sulcation of the cortex, resulting in smaller than normal gyri?
|
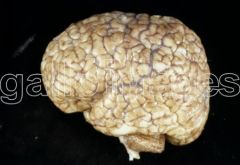
Microgyria (polymicrogyria)
|
|
|
What are the characteristics of Microgyria (Polymicrogyria)?
|
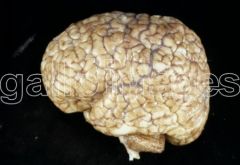
- Excessive sulcation of cortex
- Smaller than normal gyri - Only four layers in cortex due to insufficient neuroblast migration - Severe developmental delay - Speech problems - Seizures - Hypotonia |
|
|
What defect is characterized by a complete or partial absence of the corpus callosum?
|
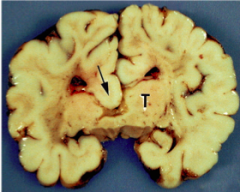
Agenesis of the Corpus Callosum
|
|
|
What are the characteristics of Agenesis of the Corpus Callosum?
|
- Complete or partial absence of the Corpus Callosum
- Can be asymptomatic - Genetic basis in some cases |
|
|
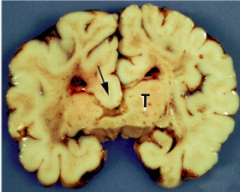
What defect is characterized by enlarged ventricles due to blockage of CSF flow and/or absorption?
|
Hydrocephalus
|
|
|
What are the characteristics of Hydrocephalus?
|
- Enlarged ventricles due to blockage of CSF flow and/or absorption
- Head will also enlarge if untreated |
|
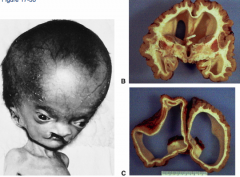
What are the two types of Hydrocephalus?
|
- Non-communicating (obstructive) - CSF can't get into subarachnoid space (usually occurs at Cerebral Aqueduct)
- Communicating (non-obstructive) - CSF can get into subarachnoid space but can't reach arachnoid granulations to be absorbed (assoc. with interventricular hemorrhage where blood break down interferes with CSF absorption) |
|
|
What characterizes the Arnold-Chiari Malformation?
|
- Associated with hydrocephalus
- Caudal displacement and herniation of ventrally located cerebellar structures (tonsils) through the forament magnum - Associated with myelomeningocele, myeloschisis, and syringomyelia (damage to spinal cord due to formation of a fluid-filled area within cord) |
|
|
What are the components of the Peripheral Nervous System?
|
- Spinal nerves and ganglia
- Cranial nerves and ganglia - Autonomic ganglia and splanchnic nerves - Sympathetic trunk - Enteric nervous system (GI system) |
|
|
What are the sensory components of the peripheral nervous system?
|
- Primary Sensory Neurons carrying sensory information from somatic and visceral organs
- Located in Spinal Ganglia and Sensory Ganglia associated with CN - Central processes are within the dorsal gray matter in spinal cord or in a CN nuclei of brainstem |
|
|
What are the motor components of the peripheral nervous system?
|
- Somatic Motor Fibers originate from neurons in ventral gray matter of spinal cord or from CN nuclei in brainstem
- Prenganglionic Autonomic Visceral Motor Fibers originate from neurons in lateral horn of spinal cord gray matter (T1-L2 for sympathetic; brainstem and S2-S4 for parasympathetic) - Postganglionic Autonomic Neurons reside in autonomic ganglia |
|
|
What parts of the Peripheral Nervous System are derived from Neural Crest?
|
- Some primary sensory neurons
- Schwann cells producing myelin sheath to surround neuronal processes - Postganglionic autonomic neurons and supporting cells of autonomic ganglia - Enteric neurons of GI tract |
|
|
What are Ectodermal Placodes?
|
- Localized thickenings of surface ectoderm found in head region of embryo
- Organized at specific locations adjacent to developing forebrain, midbrain, and hindbrain - Organization controlled by variety of genes - Contribute to CN V, VII, VIII, IX, X |
|
|
What parts of the Peripheral Nervous System are derived from Ectodermal Placodes?
|
- Primary Sensory Neurons to sensory ganglia of CN V, VII, IX, and X (come from Epipharyngeal Placodes that undergo epithelial-mesenchymal transformation and migrate toward CN)
- Receptor cells of olfactory epithelium CN I (derived from Olfactory Placodes - Sensory neurons of CN VIII (from Otic Placodes) - Retinal neurons (from Optic Disc) |
|
|
What are the primary somatic motor neurons derived from? Where do they form?
|
- Derived from neuroepithelium of neural tube
- Form in the ventral gray matter of spinal cord and brainstem |

