![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
2 Electron Densities; 0 Lone Pairs |

Linear |
|
|
3 Electron Densities; 0 Lone Pairs |
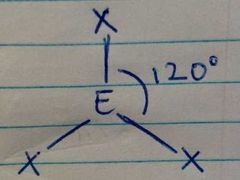
Trigonal Planar |
|
|
3 Electron Densities; 1 Lone Pair |

Bent |
|
|
4 Electron Densities; 0 Lone Pair |

Tetrahedral |
|
|
4 Electron Densities; 1 Lone Pair |

Trigonal Pyramid |
|
|
4 Electron Densities; 2 Lone Pairs |

Bent |
|
|
5 Electron Densities; 0 Lone Pair |

Trigonal Bipyramid |
|
|
5 Electron Densities; 1 Lone Pair |

Seesaw |
|
|
5 Electron Densities; 2 Lone Pairs |

T-shape |
|
|
5 Electron Densities; 3 Lone Pairs |
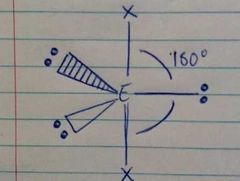
Linear |
|
|
6 Electron Densities; 0 Lone Pairs |

Octahedral |
|
|
6 Electron Densities; 1 Lone Pair |

Square Pyramid |
|
|
6 Electron Densities; 2 Lone Pairs |

Square Planar |
|
|
6 Electron Densities; 3 Lone Pairs |

T-shape |
|
|
6 Electron Densities; 4 Lone Pairs |

Linear |
|
|
Base Shape |
The shape of the molecule if only the ELECTRON DENSITIES are counted and not the lone pairs |
|
|
Actual Shape |
The shape of the molecule which includes LONE PAIRS and ELECTRON DENSITIES |

