![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
|
Matter |
-anything that has mass - takes up space -non-matters - sound - light - gravity - heat |
|
|
3 Phases of Matter |
-solid -liquid -gas |
|
|
Properties of Solids |
-fixed shape -fixed volume -low energy -no space between molecules -molecules are vibrating |
|
|
Properties of Liquids |
-flexible shape -fixed volume -medium energy -molecules are close together -room for the molecules to move around -flow in motion |
|
|
Properties of Gases |
-flexible shape -flexible volume -very high energy -molecules are far apart -random motion |
|
|
6 Phase Changes |
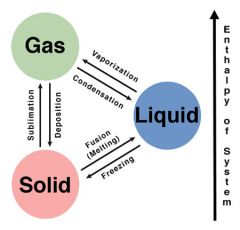
-evaporation/ vaporization -condensation -deposition -sublimation -freezing -fusion/melting |
|
|
Endothermic/Exothermic |
-endothermic - heat is being absorbed -exothermic - heat is being released |
|
|
Physical Changes |
-matter changes form but keeps the same chemical composition *usually reversible |
|
|
Chemical Changes |
-matter changes into a completely new substance with a different chemical makeup *usually irreversable |
|
|
Temperature |
-speed -a measure of the average kinetic energy in a sample -units - Celsius/Kelvin -symbol - T *conversion from Celsius to Kelvin - K=C+273 |
|
|
Heat (energy) |
-mass + speed -a measure of the total kinetic energy in a sample -unit - Joule(J) -symbol - q(quantity of energy) *common unit - calorie - 1 calorie=4.184 J |

