![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
Matter and classification |
Matter is anything that has both mass and volume (occupies space ) |
|
|
ELEMENT |
consists of only one kind of atoms |
|
|
COMPOUND |
composed of two or more elements that are chemically bound together |
|

MIXTURE |
A group of two or more substances (elements and/or compounds ) |
|
|
ATOMIC STRUCTURE |
Electrons
small,tiny particles with negative charge protons large particles with positive charge Neutrons more large and heavy with no electrical charge |
|
|
ATOMIC NUMBER |
(z) refers to number of protons in the nucleus of each atom
|
|
|
MASS NUMBER |
number of protons and neutrons in the nucleus of each atom
|
|
|
ELECTRON CONFIGRUTION |
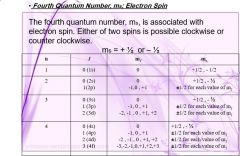
|
|
|
ATOMIC ORBITALS |
All S orbitals are serphical and differ in size |
|
|
Hybridization |
Conspet explaining how bonds are formed |
|
|
Sp3 |
Like methane |
|
|
Sp2 |
s+2p=3sp2 |
|
|
Sp |
s+p=2sp |

