![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
100 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
1. In adults, what size lymph nodes should be FNAd if no obvious explanation exists? 2. List 5 indications for lymph node FNA. 3. What are the advantages of FNA compared to surgical excision? 4. For which locations is FNA especially useful? |
1. >1-2cm (kids and adolescents this is usually reactive and often watched clinically) 2. confirm clinical impression of reactive hyperplasia diagnose suspected malignancy (hodgkin or non-hodgkin lymphoma, meatatic tumor) document a metastasis or recurrence of a known malignancy diagnose infection confirm transformation of known to higher grade lymphoma 3. rapid TAT low cost provides cells for immunophenotyping and molecular tests less morbidity preserves architecture in node should excision be necessary 4. deep nodes like mediastinal, retroperitoneal, abdominal which carry serious morbidity with excision |
|
|
|
1. Which patients may benefit the most from diagnosis by FNA? 2. What are limitations of lymph node FNA? |
1. those with oncologic emergencies (b/c of TAT e.g. spinal cord compression or airway compromise or superior vena cava syndrome), deep/surgically inaccessible nodes, advanced age or cobmorbid clinical conditions 2. sampling error (small or deep seated nodes, fibrosis, excessive necrosis or inflammation, partial involvement of node) inability to examine architecture and vascular patterns (progressive transformation of germinal centers PTGC, human immunodeficiency virus LAD, HIVAL, toxoplasma lymphadenitis, castleman disease nodular lymphocyte predominant hodgkin lymphomaT cell righ large B cell lymphoma angioimmunoblastic T cell lymphoma, DLBCL arising in follicular lymphoma |
|
|
|
1. List 4 types of romanowsky stain. 2. Why is this type of stain useful in hematopoetic neoplasms? 3. What two media are acceptable for these specimens? which additional tests can be performed? |
1. wright giemsa May Grunwald Giemsa DiffQuik Hemacolor 2. it highlights cytoplasmic details of lymphoid cells and lymphoglandular bodies AND because its the same stain used for bone marrow aspirates and peripheral blood smears so it makes for a good comparison with other heme specimens 3. RPMI-1640 cell culture OR normal saline for flow, cytospins, paraffin embedded blocks |
|
|
|
1. What is of primary importance when performing ROSE/screening these specimens? 2. What is the major relative contraindication to FNA? 3. How often does FNA create tissue artifacts and what are they? 4. What is the accuracy and PPV of lymph node FNA for metastatic tumor? 5. What is the sensitivity and specificity for non-hodgkin lymphoma on FNA? |
1. the low power pattern because it categorizes the lesion into a small cell, large cell or mixed pattern which are major branches in the DDX 2. severe coagulopathy 3. 4% of excised nodes, hemorrhage with organization and segmentl/total infarction 4. accuracy >90% and PPV 100% 5. sensitivity 80% and specificity >90% SINCE the use of flow cytometry |
|
|
|
1. List 3 lymphomas with characteristic immunophenotypes. 2. With what 3 things do ancillary studies help in diagnosing lymph node FNA? 3. List advantages of flow cytometry over IHC. 4. List advantages of IHC over flow cytometry. |
1. SLL, mantle cell and lympholastic 2. distinguish lymphoid from nonlymphoid lesions distinguish non hodgkin lymphomas from reactive lesions by confirming clonality subclassify lymphomas 3. rapid, (2 hour) turnaround time quantitative (# cells and intensity) multiple stains per cell superior detection of small monoclonal populations 4. morphology is preserved more and nuclear markers are available useful in detecting large cells including Reed-Sterberg cells |
|
|
|
SEE TABLE 12.1 ON PAGE 335 FOR COMPARISON OF FLOW CYTOMETRY AND IHC |
SEE TABLE 12.1 ON PAGE 335 FOR COMPARISON OF FLOW CYTOMETRY AND IHC |
|
|
|
1. What are the axes on flow cytometry? 2. List 3 commonly used flourochromes (which emit pulses of different colored light when excited by a lazer beam). 3. List 2 advantages of using multicolor cytometry. 4. What size specimen is sufficient for flow cytometry? include # of cells. |
1. forward scatter: proportional to cell size side scatter: proportional to cell complexity (e.g. shape of nucleus and cytoplasmic granularity) 2. fluorescein isothiocynote (FITC) phcoerythrin (PE) perdinin chlorophyll protein (PerCP) 3. fewer tubes needed multiple antigens can be examined on each cell 4. 10 aliquots of 1x10^5 cells each for a toal of 1x10^6 cells |
|
|
|
1. Which IHC is difficult to evaluation of FFPE tissue and what is an alternative? Why? 2. What |
1. kappa and lambda immunoglobulin light chains; unfixed/air dried cytocentrifuge preparations can be used but may still have significant background staining; cytospins concentrate the material into a small (often 6mm) area which conserves antibody and facilitates interpretation and is useful when there isn't enough specimen for a cell block 2. 2. |
|
|
|
SEE TABLE 12.2 ON PAGE 336 FOR IHC MARKERS IN LPD |
SEE TABLE 12.2 ON PAGE 336 FOR IHC MARKERS IN LPD |
|
|
|
1. With what do molecular genetic studies help? 2. List and describe 3 methods of accomplishing the above. |
1. identify specific DNA sequences to identify clonality (e.g T-cell) when IHC (e.g. kappa lamda ISH) fails 2. polymerase chain reaction (PCR): amplifies (with repeated denaturation/synthesis cycles, 20-60) tiny amounts of defined (flanked by known sequences, primer) regions of DNA FISH: floruochrome labeled probes for specific chromosomal regions are hybridized to intact nuclei gene expression profiling (GEP): microarray analysis |
|
|
|
1. What are the components needed for PCR? 2. What are the pros and cons of PCR? 3. For what diseases is PCR useful or not for determining clonality and why? 4. List select circumstances in which PCR is useful in the last scenario. |
1. oligonucleotide primers (complementary to DNA flanking region of interest), heat resistant DNA polymerase and the 4 nucleotides 2. Pros: fast TAT (1-2 days) no readioactive probes can be doe on FFPE tissue Cons: prone to false positive resullts requires rigorous controls requires critical review of results 3. good for T-cell receptor and immunoglobulin rearrangements but is redundant for B-cell neoplasms that show monoclonality by immunophenotyping (flow, kappa ISH stains) 4. confirmatory test when flow suggests a T-cell neoplasm when cells fail to survive flow cytometry when unfixed cells are unavailable for flow cytometry |
|
|
|
1. In what circumstances is it helpful for PCR used to detect specific breakpoints in lymphomas? 2. What must be done to make PCR more sensitive for the above and why? |
1. if the patient has a known lymphoma with a characteristic translocation and FNA is done to rule out a recurrence primary diagnosis of lymphoma if morphology suggests a specific subtype 2. more than one breakpoint must be probed because the characteristic translocations can have different breakpoints in different patients which lowers sensitivity if only one breakpoint is tested |
|
|
|
1. What kind of specimens can be used for FISH? 2. What molecular aberrations is FISH particularly good at identifying? 3. For what three tests should tissue be allocated for every case of suspected lymphoma? 4. What are potential uses of GEP? |
1. smears, cytocentrifuge, thin layer slides (especially) are optimal because the cells are intact!!! 2. translocations, deletions and amplifications (e.g. trisomies) 3. PCR, FISH and flow cytometry 4. aspirates of suspected nonhodgkin lymphoma can be rinsed in RNA stabilizing reagent for hybridizing RNA to gene chips also it maybe used for prognostic info on subtypes of DLBCL |
|
|
|
1. What are two consistent findings in aspirated lymphoid tissue? 2. What artifacts can cause pseudoclumping of lymphocytes? 3. What hematopoetic malignancy and benign condition demonstrate clumping and why? |
1. Dispersed cell pattern and lymphoglandular bodies 2. smear thickness, clotting and suboptimal spreading 3. follicular lymphoma AND lymphoid hyperplasia due to aggregates of lymphocytes adherent to follicular dendritic cells (called dendritic-lymphocytic aggregates or intact follicles) |
|
|

|
lymphoma with numerous lymphoglandular bodies 1. What are the above composed from? Describe them. |
1. detached fragments of cytoplasm; blue gray or pale blue on romanowsky stain +/- tiny vacuoles and DIFFICULT to see on pap stain! |
|

|
Reactive hyperplasia (w/o specific etiology) 1. What is the clinical picture? 2. What is the cytomorphology? |
1. children or young adults with a single moderately enlarged lymph node 2. polymorphous population small lymphs plasmacytoid lymphs centrocytes centroblasts immunoblasts tingle body macrophages dendritic cells dendritic-lymphocyte aggregates "intact follicles" capillaries eosinophils mast cells |
|
|
1. List and describe 6 of the cell types encountered in normal/reactive lymph nodes. |
1. small lymphocytes: round nuclei coarse chromatin usually the predominant cell centrocytes: intermediate sizd with irregular or cleaved nuclei, inconspicuous nucleoli and scant cytoplasm centroblasts: large cells with round vesicular nuclei, 1-3 peripheral nucleoli and narrow rim of basophilic cytoplasm immunoblasts: lare cells with fine open chromatin an done very prominent centrally nucleolus, moderate to abundant pale to basophilic cytoplasm tingle body macrophages: large phagocytic cells with round/ovoid nucleus, fine granular chromatin, small distinct nucleolus, voluminous debris-laden cytoplasm dendritic cells: large with pale oval nuclei (often bi- or multi- nucleated), small nucleoli and cytoplasmic processes (if absent cells appear epithelioid) |
|
|
|
1. What is the definition of a follicular center fragment? 2. What is the frequency of each cell type? 3. What is the DDX of reactive hyperplasia? |
1. "intact follicle" with tingible body macrophages and capillaries mixed with dendritic cells, small lymphs, centrocytes and centroblasts 2. small lymphs >> centroblasts and centrocytes > immunoblasts, plasmacytoid lymphs and tingible body macs 3. non-hodgkin lymphoma: follicular marginal zone T cell T cell rich large B cell Hodgkins post transplant lymphoproliferative disorder (PTLD) benign LAD |
|
|
|
1. In which patient populations should a diagnosis of reactive hyperplasia be avoided? 2. What are exceptions to the "heterogenous population is likely benign" rule? 3. Which benign lymphadenopathies canNOT be distinguished from reactive hyperplasia on FNA alone? |
1. elderly, those with markedly enlarge node (>3cm), deep seated nodes, or multiple enlarged nodes 2. hodgkin follicular marginal zone PTLD (EBER+) T cell lymphoma T cell rich B cell lymphoma 3. castle man disease toxoplasm alymphaenitis progressive transformation of germinal centers |
|
|
|
1. What are the 2 types of Casetleman disease (CD)? describe 2. What is the morphologic of each on tissue sections? |
1. hyaline vascular (unicentric and asymptomatic, presenting in lung and mediastinum) and plasma cell type (multicentric associated with constitutional symptoms, elevated IL6, HIV and HHV8 with peripheral LAD) 2. hyaline vascular: small hyalinized GCs and broad expansion of the mantle zone with large pleomorphic follicular dendritic cells plasma cell type: sheets of mature PCs in interfollicular areas |
|
|
|
1. What 3 features have been suggested to recognize the hyaline vascular type of CD? how reliable are these? 2. What tests can help with the diagnosis of the plasma cell type 3. What is a pitfall of the above? explain. |
1. dysplastic dendritic cells tissue fragments with branching capillaries and/or hyaline material; not specific at all 2. blood tests for HHV-8 (or IHC) and/or ILK6, 3. Because HHV-8 preferentially infects IgM lambda expressing B cells the process could appear clonal on immunophenotyping but PCR immunoglobulin gene rearrangement can demonstrate polyclonality |
|
|

|
Toxoplasma gondii 1. What is seen in this smear? what is the other form? 2. Why is the above significant? 3. What are the highly suggestive histologic features? 4. How is the diagnosis usually confirmed? |
1. bradyzoites in within enlarged granular histiocytes; free tachyzoites within an exudate 2. presence of organisms is the only way a smear can be diagnostic 3. follicular hyperplasia with small aggregates of epithelioid histocytes hugging GCs with zones of monocytoid B cell hyperplasia (none of which is seen on smears!) 4. serologic titers but PCR and IHC can be used also |
|
|
1. How is progressive transformation of germinal centers (PTGC) diagnosed on cytology? 2. What is it and what is a unique finding? 3. List other entities with the above "unique" finding. 4. What occurs in this disease histologically? 5. What happens to patients with this? |
1. It's not! 2. a significant proportion of double positive (CD4 and CD8) T cells on flow cytometry 3. T cell lymphoma thymoma nodular lymphocyte predominant hodgkin lymphoma (NLPHL) 4. follicle expansion and disruption with replacement by mantle cells (small to intermediate lymphs with roun /irregular nuclei, inconcpicuous nucleoli and scant cytoplasm) 5. most do NOT develop lymphoma |
|
|
|
Sarcoidosis 1. What is the patient population and most commonly involved sites? 2. What is an important practical point of late phase sarcoidosis? 3. What is the cytomorphology? 4. What are the key nuclear features? |
1. young to middle aged adults more so in African Americans in lung and mediastinal lymph nodes >> peripheral nodes especially head and neck 2. hypocellular FNA with scant granulomas due to fibrosis 3. non-caseating granulomas epithelioid histiocytes multinucleated giant cells lymphocytes hypocellular aspirate (late phase) clear background 4. elongated, elliptical, spindle shaped curved or sligntly indented nucleus with "footprint, C-shaped, V-shaped or boomerang" |
|
|
1. What is the DDX of sardoidosis? List examples of some. |
1, infection: fungal, mycobacterial> bacterial foreign body reaction spindle cell neoplasm malignancies with granulomas: Hodgkin, T cell lymphoma, SQC and seminoma dendritic lymphocytic aggregates 2. |
|
|

|
Acute bacterial lymphadenitis 1. What is the cytomorphology? 2. What should be done with any specimen that looks like pus? 3. How does fungal lymphadenitis differ from bacterial? |
1. high cellularity composed of almost all neutrophils with variable degeneration 2 submit for culture in sterile container or culturette device 3. variable morphology of inflammatory infiltrate (neutrophils only, granulomas only or a mix and sometimes no reaction to the organisms!) |
|
|
1. What are the more common fungi encountered in FNA? 2 What is needed for definitive diagnosis of the above? 3. Why is identification of organisms important? |
1. cryptococcus neoformans histoplasma capsultum coccidoides immitis 2. culture and stains (GMS, PAS mucicarmine for cryptococcus capsule) which can be performed on cell block or destained/unstained smears 3. treatment selection AND excluding malignancy |
|
|

|
Cryptococcus neoformans 1. What stain is used to highlight this characteristic feature? |
1. mucicarmine to highlight the capsule!
|
|

|
Histoplasma capsulatum 1. Where is the organism commonly found? |
1. in macrophages and organs with a prominent lymphoepithelial system like liver and spleen
|
|

|
Coccidoides immitis |
|
|
|
1. What is the causative organism of cat scratch disease? how often is there an associated bite or scratch from a cat? 2. What is the primary patient population, location and prognosis? 3. What are the histologic and cytologic hallmarks of this diseases? 4. What method can be used to stain them and how reliable is it? 5. What is the DDX? 6. List other organisms that produce an identical cytologic picture. |
1. Bartonella henslae; 50-75% of the time 2. kids/teens in nodes of the axilla, inguinal region and neck that is self limited; resolving in 4-6 months 3. histologic: stellate microabscesses cytologic: neutrophils with loose or tight granulomas and necrosis 4. Steiner method but highly variable results 5. other causes of suppurative granulomatous lymphadenitis reactive lymphadenitis 6. Franciscella tularensis chlamydia trachomais yersinia enterocolitica yersinia pseudotuberculosis > M. tuberculosis |
|
|

|
Mycobacterial lymphadenitis 1. Which patients are affected by these infections? 2. What is the cytomorphology? 3. What phenomenon is seen in the photo? explain why and in which organisms. 4. How is the above phenomenon seen on pap stain? |
1. immunocompetent and immunosupressed (especially HIV+ patients)
2. necrosis granulomas (may be only histiocytes if immunocompromised!) hitocytes neutrophils intra and extra cellular bacilli "negative images" acid-fast staining bacilli 3. negative images; seen in nontuberculous mycobacterial infections typically M. avium complex due to the lipid coat of the bacilli resisting stain with any romanowsky stain and appear as optically clear rods/striations within macrophages (resemble tissue paper Gaucher cells) or extracellular 4. It's not visible! |
|

|
Mycobacterium avium intracellulare 1. What kind of stain is this? 2. What i the DDX of mycobacterial lymphadenitis? 3. What is the sensitivity for special stains? be organism specific. 4. What is an alternative way to diagnose these infections? |
1. Ziehl-Neelsen 2. sarcoidosis granulomatous lymphadenitis due to other organisms lymph node infarction (SLE, lymphoma, vascular thrombosis, trauma and infection) 3. low especially for M. tuberculosis 4. molecular techniques (detect and speciate mycobacteria!) |
|

|
Rosai dorfman disease (RDD) 1. What is the patient population and presentation? 2. What are the lab findings? 3. What is the cytomorphology? 4. What is the IHC profile? 5. What is the DDX? |
1. children/teens with bilateral painless cervical lymphadenopathy, fever, joint pain, night sweats and weight loss which mimics lymphoma 2. polyclonal hypergammaglobulinemia and leukocytosis 3. lots of small lymphocytes histiocytes with emperipolesis 4. histocytes are S100 and CD68 positive 5. reactive lympoid hyperplasia langerhans cell histiocytosis |
|
|
1. What is an artifact that can mess up the characteristic finding of RDD? 2. How is Langerhans cell histiocytosis differentiated from RDD? |
1. lymphocyte "spill" due to smearing mechanism 2. uncommonly involves lymph nodes withOUT simultaneously involving another site like lung or bone neoplastic cells CD1a + (in addition to S100 and CD68) histiocytes with contorted, reniform nuclei, grooves and no nucleoli (unlike round nuclei of RDD) no emperipolesis increased eosinophils |
|
|

|
Kikuchi lymphadenitis 1. AKA? prognosis, patient population and presentation? 2. What is the cytomorphology? 3. How are the characteristic cells differentiated from tingible body macrophages (TBMs)? 4. What is the DDX? 5. What cell is helpful to distinguish this disease from SLE? |
1. histocytic necrotizing lymphadenitis; self limiting in young Asians with cervical LAD and tenderness, fever, and atypical peripheral lymphocytosis 2. necrotic debris karyorrhexis small phagocytic histiocytes with sharp angulated peripheral "crescent-shaped" nuclei cytoplasmic tingible bodies increased immunoblasts and plasmacytoid monocytes NO neutrophils 3. TBMs have round, central nuclei and are larger 4. reactive follicular hyperplasia (TBMs) tuberculous lymphadenitis necrotizing supperative granulomatous lymphadenitis SLE 5. hematoxylin bodies (darkly stained clumps of nuclear debris) seen in lupus |
|
|
1. What is the causative organism and cytomorphology of infectious mononuceosis? 2. What does immunophenotyping often show? 3. What is the DDX? 4. What other conditions cause immunoblast proliferations indistinguishable from this? |
1. EBV; increased immunoblasts, plasmacytoid lymphocytes and plasma cells few dendritic lymphocytic aggregates and TBMs 2. reversed CD4/CD8 ratio (normal is 2x cd4 to cd8) 3. reactive lymphoid hyperplasia LAD with increased immunoblasts large cell lymphoma hodgkin lymphoma 4. anticonvulsant associated (usually phenytoin) herpes simmplex CMV post vaccine drug hypersensitivity |
|
|
|
1. What is the cytomorphologic pattern of HIV associated LAD? is this diagnostic? 2. In HIV patients when is FNA most useful? 3. What are the histologic changes of HIV LAD not seen on FNA? |
1. early changes of striking follicular hyperplasia pattern shows "giant" intact follicles, large cells and plasma cells on FNA;no it's a diagnosis of exclusion 2. identification of infections and neoplastic causes of LAD 3. mid-phase: follicular involution, partial lymphocyte depletion and castleman like changes late-phase: small depleted follicles and paracortical vascular hyperplasia |
|
|
|
1. What are the causes of dermatopathic lymphadenitis? 2. What cytologic findings are suggestive of this? 3. What test is used to distinguish this from invovlement by mycosis fungoides? |
1. (nonspecific lymphadenitis) chronic dermatoses (psoriatic erythroderma, exfoliative dermatitides and mycosis fungoides) and skin/tissue injury or foreign material (e.g. tatoos) 2. numerous dendritic cells and pigment laden macrophages withOUT TBMs!! 3. T cell receptor gene rearrangement |
|
|

|
Silicone lymphadenitis 1. What is the cytomorphology? |
1. large multinucleate giant cells with vacuoles containing hyaline material (on direct smears) or empty appearing vacuoles (after processing) |
|
|
1. What is required from the cytopathologist for FNA to be a valuable too in primary diagnosis/classification of lymphoma? 2. What 4 things define lymphomas? which is of less importance currently which is of benefit to FNA diagnosis? 3. What is included in a comprehensive FNA lymph node workup? |
1. understanding of modern lymphoma classification using special studies (immuopheontyping and molecular genetics) works closely with a hematopathologist 2. morphology, immunophenotype, genetics and clinical findings; architectural pattern is of less importance than previous 3. clinical history rapid evaluation good smears needle rinse (flow cytometry, IHC, molecular studies, tissue fragments for architecture or IHC) |
|
|
|
1. What is the WHO classification for Hodgkin lymphoma? 2. How common is this lymphoma? 3. What do the two main groups have in common? 4. For the more common type, what is the patient population? |
1. Classical 95% Nodular sclerosis lymphocyte rich mixed cellularity lymphocyte depleted Nodular lymphocyte predominant 2. accounts for 30%!!! 3. the neoplastic cells are outnumbered by non-neoplastic background inflammatory cells 4. Bimodal distribution with a large peak in 15-35 yrs and smaller peak later in life |
|
|
|
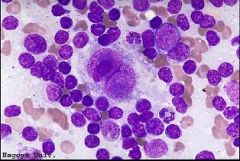
Reed sterberg cell (RS cell) 1. What are they? 2. What is the usual immunophenotype? 3. Importantly what stain is negative in RS cells? |
1. monoclonal B cells classically binucleate with enormous nucleoli (mononuclear variants are common) 2. CD30+ (majority but non specific) and CD15+ (75-85%, difficult focal RS staining and background granulocyte positivity) PAX5/BSAP+ (95%) minority weak CD20+ (<40%) 3. CD45! which is difficult to interpret in the background of abundant positive lymphocytes |
|
|
1. What is the clinical relevance of separating the 4 histologic variants of classical hodgkin lymphoma? 2. What are the clinical, morphologic and IHC features of nonclassical hodgkin lymphoma (NLPHL)? 3. What is on the DDX of this type that makes FNA less useful and why? |
1. NONE 2. clinical: male patients 30-50 years with cervical, axillary or inguinal LAD (rather than classical mediastinal) morphologic: L&H cells are large monoclonal B cells with one large folded or multilobate nucleus "popcorn cells" with smaller nucleoli IHC: CD20+ and CD45+ and 50% are EMA+ (compared to 5 3. T cell rich LBCL; NLPHL is distinguished for its nodular architecture which is not appreciated on FNA |
|
|
|
1. What is the cytomorphology of hodgkin? 2. What flow cytometry finding is seen in a significant proportion of NLPHL? 3. What is the DDX of hodgkin lymphoma? |
1. small lymphocytes eosinophils (especially mixed cellularity) RS cells (classic and the more common mononuclear variants) L&H cells (in the rare NLPHL) NO follicular aggregates or TBMs (exceptions: lymphocyte predominant type and partial node involvement) 2. double positive (CD4+/CD8+) T cells (also seen in PTGC and T cell lymphomas) 3. reactive lymphoid hyperplasia EBV TCR-LBCL anaplastic large cell lymphoma (ALCL) acute lymphadenitis (rare) nasopharyngeal carcioma (NPC) |
|
|
|
1. What is a major pitfall in diagnosis of Hodgkin lymphoma on FNA? why? 2. What reactive cell do mononuclear RS cells resemble? What stain is NOT helpful in this situation? 3. What is helpful in differentiating ALCL from hodgkin on FNA? |
1. hypocellularity; because nodular sclerosis is the most common subtype and may result in insufficient RS cells 2. immuoblasts (e.g. in EBV infection); EBERish because it can be positive in both 3. ALCL often has numerous neoplastic cells (versus 0.1-10% malignant cells in Hodgkins) |
|
|
|
1. What can differentiate Hodgkin and ALCL in difficult cases? 2. What can distinguish metastatic nasopharyngeal carcinoma from Hodgkin? 3. What is the frequency of non-hodgkin lymphomas? |
1. stains; EMA + in ALCL CD30/CD15/BSAP + in Hodgkin 2. cytokeratin 3. 90% B cell: 2/3 are DLBCL and follicular 10% T cell small minority are null cell 4. |
|
|
|
SEE TABLE 12.4 ON PAGE 350 FOR WHO CLASSIFICATION OF B-CELL NEOPLASMS |
SEE TABLE 12.4 ON PAGE 350 FOR WHO CLASSIFICATION OF B-CELL NEOPLASMS |
|
|
|
SEE TABLE 12.5 ON PAGE 350 FOR CLINICAL FEATURES OF SMALL B-CELL LYMPHOMAS |
SEE TABLE 12.5 ON PAGE 350 FOR CLINICAL FEATURES OF SMALL B-CELL LYMPHOMAS |
|
|

|
Follicular lymphoma (FL, grade 1) 1. How common is this? 2. What is the patient population and presentation? 3. What is the characteristic immunophenotype? 4. What is the characteristic molecular alteration and how is it identified? |
1. 35% of adult non-hodgkin lymphoma 2. >50years; 80% present with disseminated disease (spleen, multiple lymph nodes, bone marrow), 25-35% trasnform to LBCL (which is rapidly progressive and resistant to treatment) 3. CD10+, BCL6+(reliable, nuclear) and CD51; BUT 40% are CD10-!!! especially on flow alone 4. t(14;18) with rearrangement of (IgH and) BCL2 gene in 95% of cases, detected 80% of time by FISH (making FISH more sensitive than flow for dx of FL) |
|
|
1. What is the cytomorphology of FL? 2. List variants of FL. What is the significance? 3. What are the two categories of FL? based on? |
1. predominantly small irregular/cleaved lymphocytes large cleaved/non cleaved lymphocytes (in high grade) few TBMs lymphoid cell aggregates (>1/3 of cases) 2. with monocytoid B cells with rosettes floral pattern with cerebriform nuclei signet ring cell NO clinical significance 3. Low grade: WHO 1-2, favored on FNA if the cells below are <40% High grade: WHO 3, not distinguishabel from DLBCL on FNA but may not be clinically relavant anyway based on the number of centroblasts (large nucleolated cells) on TISSUE sections |
|
|
|
1. What are the types, commonality and prognosis? 2. In what FNA specimens do cytolgists see this? 3. With what two autoimmune diseases is this associated? 4. With what infections is it associated? 5. What are the molecular alterations? |
1. indolent low grade, often present with stage 1-2 disease which is potentially curable w/ surgery or radiation nodal: extra nodal: MALT lymphoma, more common, 7-8% of all B cell lymphomas 2. salivary gland, lung, lacrimal gland, thyroid, breat and skin 3. Hashimoto thyroiditis Sjogren syndrome 4. H. pylori, Chlamydia psistacci, campylobacter jejuni 5. too many to be helpful on FNA which vary by site (t11;18 API2 and MALT, trisomy 3 or trisomy 18) |
|
|
|
1. What is the characteristic lesion? 2. What is the cytomorphology? 3. Immunoprofile? |
1. lymphoepithelial lesion with invasion of the epithelium with nests of neoplastic cells 2. polymorphous population small lymphocytes round (slightly irregular) nuclei monocytoid cells (moderate amount of pale cytoplasm) plasma cells follicular dendritic cells, TBMs, follicular aggregates and immunoblasts (reactive elements) 3. CD5- CD10- |
|
|
|
SEE TABLE 12.6 ON PAGE 351 FOR DIFFERENTIAL IHC AND GENETIC OF SMALL B CELL LYMPHOMAS |
SEE TABLE 12.6 ON PAGE 351 FOR DIFFERENTIAL IHC AND GENETIC OF SMALL B CELL LYMPHOMAS |
|
|

|
Small lymphocytic lymphoma 1. How common is this? 2. what is the prognosis and alternate name? 3. What is the highly characterisitc immunophenotype? |
1. 6% of non-hodgkin lymphomas 2. indolent, incurable, widespread (nodal and extgranodal) usually involving the peripheral blood and bone marrow (called chronic lymphocytic leukemia) 3. CD5+, CD23+ and CD10- KNOWING that CD5 and CD20 expression are dim/weak |
|
|
1. What is the cytomorphology? 2. How common is transformation to DLBCL? 3. What findings are seen in the above? 4. What other transformation can occur? |
1. monomorphous small lymphocytes clumped (soccer ball like or clotted) chromatin (especially on pap!) smooth nuclear contour scant cytoplasm smudge cells absent/inconspicuous nucleoli prolymphocytes and paraimmunoblasts (create the proliferation centers on tissue sections) rare/absent TBCs and follicular aggregates 2. up to 20%; clinical concern for this is the most common indication for an FNA 3. large cells, necrosis and KI-67 index >30% 4. to hodgkin! |
|
|

|
Mantle cell lymphoma 1. How common is this and what is the prognosis? 2. Patient population? 3. What is the characteristic molecular alteration and physiology? 4. What is the immunophenotype/ |
1. 3-10% of non-hodgkin B-cell lymphomas; agressive, commonly extranodal with disseminated disease and subtle peripheral blood involvment at presentation; resistant to therapy with median survival of 3-5 years 2. adults >50 years, M>F 3. t(11;14) (q13;q32) of IgH and BCL1 resulting in overexprfession of cyclin D1 which acts on G1 to S phase checkpoint and drives cell into proliferation 4. CD5+, CD23-, CD10- |
|
|
1. What is the cytomorphology of mantle cell? 2. What is the "rule" morphologically compared to other small cell lymphomas? 3. How often does the characteristic immunophenotype vary? what is a solution? |
1. monomorphous small to intermediate lymphocytes fine chromatin irregular nuclear contours (subtle clefts) inconcspicuous/absent nucleoli scant cytoplasm (high N:C ratio) no centroblasts or immunoblasts "pink" histocytes (moderately abundant eosinophilic cytoplasm) lymphoid cell aggregates (1/3 of cases) blastoid variant 2. monotony b/c NO transformed lymphocytes! 3. 10-20%, demonstrate cyclin D1 reactivity or t(11;14 by FISH) |
|
|
|
1. What is the DDX for small cell lymphomas? 2. What finding is suggestive of NOT lymphoma? what is the exception? 3. Which small cell lymphoma is an exception to the rule of morphologic homogeneity? why? 4. Which small cell lymphomas are more likely to have extranodal location? |
1. reactive hyperplasia hodgkin follicular MZS/MALT SLL MCL 2. dendritic lymphocytic aggregates with TBMs (follicle clusters) is seen in reactive hyperplasia but NOT small lymphomas EXCEPT follicular lymphoma 3. MZL, b/c of its associated nonneoplastic polymorphous infiltrate of small lymphs, centrocytes and monocytoid B cells also PCs and plasmacytoid cells 4. MZL and MCL > FL (especially head and neck) but rarely SLL |
|
|
|
1. Give an algorithm for the small cell lymphomas based on IHC. 2. What does SLL characteristically express weakly/dim? 3. Which marker is often negative in FL and by what modality? 4. What are the molecular aberrations in CLL? 5. What are the hallmark molecular alterations for the other small cell lymphomas? |
1. CD5+: (SLL and MCL) CD23+ SLL CD23- MCL (BCL1+ highly specific) CD5- (FL and MZL) CD10+ FL (23+/-) CD10- unresolved 2. CD20 and light chains 3. CD10 often negative on flow cytometry which is why a CD5- and CD10- must have IHC for CD10 and bcl-6 to distinguish FL from MZL (or molecular) 4. trisomy 12 (30%) del13q14.3 del11q22-23 del17p13 5. MCL t(11;14) (q13;q32) FL t(14;18) (q32;q21) (95% of cases) |
|
|
|
1. What disease dominates the large cell lymphoma category? 2. What is a difference in the patient population for large (versus small) cell lymphomas? 3. What is the cell of comparison for "large" cell lymphomas? |
1. DLBCL though other origins include T cells or NK cells 2. large often occur in kids in addition to adults; most common in kids are DLBCL, lymphoblastic lymphoma and Burkitt lymphoma 3. histocyte nuclei (which means they're often smaller than epithelial nuclei) |
|
|

|
DLBCL 1. How common? usual presentation? 2. What are the histologic subtypes of DLBCL? 3. How is GEP helpful? 4. List two common gene rearrangements and their frequency. |
1. 35% of adult lymphomas and 1/3 major kid lymphomas;40% present with extranodal disease and although aggressive it's potentially curable! 2. centroblastic, immunoblastic and anaplastic NOT clinically relavant 3. it separates germinal center B-cell like and activated B-cell like subtypes of DLBCL (helps with survival prediction after chemo) 4. BCL2 rearrangement, 30% abnormal BCL6 on 3q27, 30% |
|
|
1. What is the cytomorphology of large B cell lymphoma? 2. DLBCL in which locations are prone to hypocellularity and why? 3. What are two pitfalls of large cell lymphoma on flow cytometry? |
1. predominantly large cells (2.5-5x size of small lymph; nucleus larger than histiocyte nucleus) distinct to large nucleoli lymphoglandular bodies variable TBMs paucity or absence of dendritic-lymphocytic aggregates 2. mediastinal and extranodal due to extensive sclerosis or sparse distribution of neoplastic cells 3. the atypical cells may fall outside the "lymphoid gate" or may not survive processing due to fragility |
|
|
|
1. What other method to identify clonality can be unpredictable in DLBCL? 2. How does DLBCL stain for B cell markers? |
1. light chain expression b/c they often LACK it! (but that in itself is abnormal so a clue!) may need PCR to determine clonality 2. bright CD20 on flow and IHC UNLESS s/p rituxumab then PAX5/BSAP can be used |
|
|
|
1. List and describe variants of DLBCL. |
1. primary mediastinal/thymic: young adult females, tumor expresses CD30, IRF4/MUM1, TRAF1 and distinctive nuclear c-Rel double hit: both IgH-bcl-2 and Cmyc translocations; high incidence in older patients with advanced/aggressive disease and poor prognosis T-cell/histocyte rich: very few neoplastic cells, not diagnosed on FNA commonly primary effusion lymphoma intravascular LBCL |
|
|
|
SEE TABLE 12.7 FOR DIFFERENTIAL FEATURES OF DLBCL AND MIMICS |
SEE TABLE 12.7 FOR DIFFERENTIAL FEATURES OF DLBCL AND MIMICS |
|
|
|
1. What other molecular alterations can be seen in double hit lymphoma? 2. With what other lymphoma does double hit share similar morphology and IHC expression and what are some differences? |
1. three way translocation (8;14;18) with bcl-2, IgH and c-myc; bcl-6 and c-myc translocation 2. burkitts; but double hit shows expression of bcl-1 and only moderately high KI67 and sometimes reduced CD20 on flow cytometry |
|
|

|
Burkitt lymphoma 1. What is the prognosis and cause ? 2. Why is this diagnosis especially important? 3. In what 3 clinical settings does this disease occur? |
1. highly aggressive but potentially curable; constitutively activated c-myc oncogene 2. it necessitates more aggressive chemo regimen than other b cell lymphomas 3. endemic: africa/asia, kids, ALL EBV associated sporadic: US, kids, subet EBV associated immunodeficiency associated: adults, subset EBV associated |
|
|
1. What sites does Burkitt involve? 2. How commonly is CSF involved at presentation? 3. What is the cytomorphology? |
1. lymph nodes and or extranodal tissue (GI, liver and bone marrow common in NON endemic form) 2. 20-30% 3. uniform intermediate sized cells round nuclei with coarse chromatin 2-5 small nucleoli per nucleus apotosis and numerous mitoses scant blue cytoplasm with small vacuoles (romanowsky stain) numerous TBMs |
|
|
|
1. What is the buzz word for Burkitt's and what causes it? 2. What feature is commonly seen in DLBCL? 3. List +IHC in Burkitts. 4. What is the molecular alteration? 5. How is the above most reliably identified and what is an alternative? 5. What is the finding in FISH compared to all other translocations previously discussed? |
1. starry sky pattern due to TBMs randomly dispersed though the monotonous hypercellular smear 2. lipid vacuoles 3. + CD19 C20 IgH, monotypic surface light chains, CD10 and BCL6 and 100% Ki67 (- BCL2 and TdT) 4. c-myc (on 8) translocation with heavy chain (14) or kappa/lambda light chains (2/22) 5. cytogenetics but FISH can and nuclear c-myc positivity are suggestive 6. it shows a split c-myc signal (rather than fusion of two normal) indicating rearrangement |
|
|
|
1. What is the general sensitivity and specificity of c-myc arrangements for Burkitt's lymphoma? |
1. its highly sensitive but not specific as it's seen in DLBCL > FL, late-stage PCM and unclassifiable with features intermediate between |
|
|

|
Plasmablastic lymphoma 1. In what patient population does this occur and how common is it? 2. What is the common location at presentation? 3. What is unique about this neoplasm? 4. What is the immunophenotype? |
1. HIV+ or other immunodeficient patients, rare 2. oral cavity and other mucosal sites but also lymph nodes 3. morphologically resembles B immunoblasts (large w/ large central nucleoli) but IHC like plasma cells 4. positive: CD138, CD38, IRF4/MUM1 and EBER ISH negative: CD45, CD20, PAX5/BSAP and CD56 (unlike PCM) |
|
|
1. How common are T cell lymphomas? 2. What are the two main broad categories and what is unique about T cell compared to B cell lymphomas? 3. Which T cell lymphoma has a specific genetic association? |
1. 10% of all non-Hodgkin lymphomas 2. precursor T lymphoblastic and mature T/NK cell; characterization is based on clinical features not immunprofile (like B cell) because most are non specific and most do NOT have specific genetic changes 3. Anaplastic large cell t(2;5) |
|
|
|
SEE TABLE 12.8 ON PAGE 360 FOR WHO CLASSIFICATION OF T AND NK CELL NEOPLASMS |
SEE TABLE 12.8 ON PAGE 360 FOR WHO CLASSIFICATION OF T AND NK CELL NEOPLASMS |
|
|
|
1. What are the antigenic markers for clonality in T cell lymphomas (e.g. kappa lambda in B cell)? 2. What IHC changes suggest T cell lymphoma? 3. What two neoplasms can mimic T cell lymphoma and why? 4. What is often required to confirm clonality of T cell proliferation? |
1. there are none!!! 2. abberrancies like: loss of 1+ T cell markers (CD2, CD3, CD5, CD7 or CD4&8) coexpression of CD4&8 3. NLPHL: background lymphs may coexpress CD4/8 Thymoma: background lymphs can be double +/- for CD4/8 4. most commonly PCR for T cell receptor gene rearrangement |
|
|
|
1. What are the most common mature T cell lymphomas encountered by a cytologist? 2. Where is peripheral T cell lymphoma of unspecified type much more common than in the US? 3. What is the patient population in the US? 4. What is the cytomorphology? |
1. peripheral T cell, unspecified ALCL mycosis fungoides (with lymph node involvement) adult T cell leukemia/lymphoma 2. Asia>>>Europe and North America 3. elderly and systemically ill with fevers night sweats and bulky LAD 4. monomorphous small or large lymphocytes (or a mix) irregular nuclei histocytes, plasma cells and eosinophils (70% cases are polymorphous> large cell or mall cell predominant) Reed-Sternberg like cells |
|
|

|
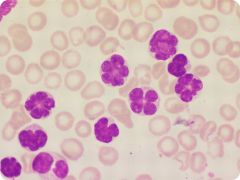
Analplastic large cell lymphoma (ALCL) 1. How common is this? 2. Name and describe the characteristic cell seen in this photo (and classic ALCL). 3. What are two variants? |
1. 3% of non-Hodgkin lymphomas and 10-30% of childhood lymphomas 2. "hallmark cell" which is larbe and bizarre with a horseshoe shaped nucleus 3. lymphohistiocytic and small cell |
|
|
1. What two genes are commonly rearranged in ALC? 2. Which patient population often lacks the above? 3. What stain is helpful in diagnosis and prognosis and how? 4. What is the typical immunoprofile? 5. These are typically negative for? 4. What is the cytomorphology? |
1. ALK and nucleophosmin (NPM) in the t(2;5) 2. adults 3. ALK (+ in 60-85%); ALK+ have better prognosis than ALK- 3. intermediate and large cells irregular nuclei: horseshoe, donut and embryoid reed-Sternberg like cells with smaller nucleoli histocytes and neutrophils +/- necrosis NO TBMs or dendritic lymphocytic aggregates few/absent lymphoglandular bodies 4. positive for CD30 (golgi and membranous pattern) ALK (can use FISH breakapart), EMA and clusterin 5. EBV RNA |
|
|
|
1. What is the endemic regions of adult T cell leukemia/lymphoma? 2. What is the cause? 3. What is the characteristic (but not necessarily prominent) cell pictured? 4. What are the characteristic clinical features required for diagnosis? |
1. Caribbean islands of western hemisphere, southern Japan, Africa, Latin America 2. HTLV-1 retrovirus 3. floret cell with "floretlike" multilobulated nuclei, prominent nucleoli and deeply basophilic cytoplasm with occasional vacuoles 4. ethnic background, hypercalcemia, atypical lymphocytosis in peripheral blood, coupled with flow cytometry (+ CD2/3/4/5/25 and - CD7/8) with seroligic testing for HTLV-1 |
|
|
1. What is the prognosis, patient population and location of lymphoblastic lymphoma? 2. Why is FNA the method of choice for this diagnosis? |
1. aggressive compromising 50% of childhood non hodgkin lymphomas, M>F, almost always above the diaphragm with 80% having an anterior mediastinal mass 2. Because of the expediency as many present critically ill (neck mass with respiratory compromise mimicking asthma or superior vena cava syndrome) |
|
|

|
lymphoblastic lymphoma 1. What is the cytomorphology? 2. What percent are of T cell derivation? 3. What is the immunophenotype? 4. What helps distinguish from thymoma? |
1. monotonous cells round or convoluted nuclei finely granular chromatin inconspicuous nucleoli scant or moderate cytoplasm 2. 90%!!! 3. + Tdt and CD10 - light chains 4. clinical presentation, keratin positive cells in thymoma and mature appearing lymphs (but IHC mimic) |
|
|
1. How common are post transplant lymphoproliferative disorders? 2. How commonly is there a viral association and which one? 3. What sites do they involve and what is the treatment? |
1. occur in 1-2% of solid organ and bone marrow transplants 2. 80% are associated with EBV 3. usually lymph node and GI tract; treat with decreasing immune suppression which usually causes regression otherwise chemo is used |
|
|
|
1. What are 4 histologic categories of PTLDs? Describe. |
1. early: polyclonal, non-neoplastic proliferations that preserve node architecture and show plasma cell hyperplasia (EBV like) polymorphic: destructive, neoplastic proliferation of all B-cell stages, flow may appear plolytypic but molecular show rearrangments of IgH and monoclonal episomal EBV monomorphic: destructive, neoplastic proliferation of large cytologically malignant B cells with prominent nucleoli resembling non-hodgkin (a few can be T cell origin) hodgkin/-like lymphoma: |
|
|
|
1. How can early and polymorphic PTLD be differentiated on cytology? 2. What is the cytomorphology of monoclonal lesions and how are they classified? why? |
1. they can't, overlapping features of mixed cellularity but needs molecular to distinguish clonality (and polymorphic has many EBV+ cells) 2. cytologically malignant, classified by WHO 2008 like regular lymphomas BUT must mention the term PTLD b/c it gives the treatment option of immunosuppression reduction 3. |
|
|
|
1. Describe light chain restrictions in PTLDs. 2. What stain is helpful in this diagnosis? 3. What percent are negative for the above? |
1. often ABSENT by blow and IHC in poly- and mono- morphic (so absence doesn't exclude PTLDs) requiring molecular testing to demonstrate clonality 2. ISH for EBV encoded RNA (EBER); a positive result in the majority of the lesional cells strongly supports the diagnosis of PTLD. 3. 20% (so patients w/ bone marrow transplants the DDX includes recurrent lymphoma) |
|
|
|
1. What is the DDX of lrage cell lymphoma? HUGE 2. What is a big clue to carcinomas mimicking large cell lymphomas? list two exceptions. |
1. nonhematopoetic tumors reactive hyperplasia Hodgkin DLBCL Burkitt or Burkitt-like Double Hit lymphoma peripheral T cell NOS ALCL precurser T- and B-cell Thymoma PTLD myeloid sarcoma histiocytic neoplasms dendritic neoplasms 2. LACK of lymphoglandular bodies! nasophagyngeal carcinoma and seminoma 3. |
|
|
|
1. What makes ALCL a very good mimic of carcinoma? 2. Which non-hematopoetic tumors are in the DDX of lymphoblastic and Burkitt's lymphoma and why? 3. What cytomorphologic features help distinguish the above? |
1. paucity of lymphoglandular bodies cell clustering marked pleomorphism spindle cells immunoreactivity for EMA scant smaller lymphocytes some are negative for CD45 (but + for CD3, CD30, clusterin and/or ALK) 2. they are common in kids and have an intermediate cell size which adds SRBC malignancies to the DDX (e.g. neuroblastoma, rhabdomyosarcoma, Ewing sarcoma/PNET) 3. nonhematopoetic: lack lymphoglandular bodies form aggregates in smears neuroblastoma forms rosettes, neuropil and nuclear molding rhabdomyosarcoma has more anisonucleosis, abundant tapered cytoplasm often bi- or multinucleated |
|
|
|
1. What is a clue to T cell lymphoma on cytology? 2. What is a pitfall of mediastinal DLBCL? 3. In what patient population should one be especially careful of the above scenario? |
1. nuclear membrane irregularities (indentations, grooves, knobs) even with mixed small/large cells that may mimic hyperplasia 2. extensive sclerosis leads to sparsely cellular smears often with crushed lymphoid cells and fibrous tissue fragments that mimic spindle cell neoplasms and granulomatous inflammation 3. young adult females |
|
|
|
1. Which 3 lymphomas usually require immunophenotyping to be differentiated? 2. What is the main differential of lymphoblastic lymphoma and why? 3. What is the characteristic clinical presentations of subtypes of Burkitt lymphoma? 4. What is the characteristic IHC of Burkitts? |
1. peripheral T-cell (large cell type) DLBCL (especially T-cell/histocyte rich) and Hodgkin; the former are morphologically identical 2. Thymoma because the lymphocytes are also Tdt positive (flow and CK stains needed) 3. endemic: jaw/orbit sporadic: abdomen HIV associated: other extranodal sites (this is the main point for all of these) 4. B-cell antigens, CD10 and BCL1 while negative for BCL2 (+ DLBCL) and Tdt (+ lymphoblastic lymphoma) |
|
|
|
1. What is a clue to a myeloid sarcoma that mimics lymphoma? 2. What must occur once a myeloid neoplasm is diagnosed and why? 3. How common are dendritic and histiocytic cell neoplasms? |
1. flow cytometry with dim CD45 (follwo up with myeloid markers, CD13/33/117 or IHC for MPO cloroacetate esterase is necessary) 2. full genetic analysis with cytogenetics, FISH or other molecular assays b/c to stratify them into prognostically and therapeutically relevant subgroups 3. rare, <1% of of tumors in lymph nodes |
|
|
|
SEE TABLE 12.9 ON PAGE 366 FOR HISTIOCYTIC AND DENDRITIC CELL NEOPLASMS/MARKERS |
SEE TABLE 12.9 ON PAGE 366 FOR HISTIOCYTIC AND DENDRITIC CELL NEOPLASMS/MARKERS |
|
|
|
1. What does histiocytic sarcoma morphologically resemble? 2. What distinguishes the above and what overlaps? 3. List function and types/locations of dendritic cells. 4. For what stain are the above positivt? |
1. DLBCL and ALCL 2. histiocytic sarcoma is CD68/163+ but myeloid lesions are also so you need negativity for myeloid and immature markers to exclude those 3. antigen presenting cells Langerhans: skin interdigitating: lymph nodes follicular: follicles of lymph nodes 4. S100 |
|
|
|
1. How is langerhans cell histiocytosis recognized? 2. What is the clinical presentation? 3. What is the characteristic immunophenotpye of the above cell? |
1. Langerhans cell: grooved, indented or lobulated vesicular nucleus and abundant (epithelioid) cytoplasms with background eosinophils, histiocytes, neutrophils and lymphocytes 2. kids or adults, unifocal or multifocal commonly in bones, skin and lungs 3. + CD1a and langerin (and S100) |
|
|
|
1. What is the immunoprofile of the extremely rare interdigitating dendritic cell sarcoma/tumor? 2. Which stain is best to identify myeloid blasts and why? 3. What is the principal mimic of SmCC of the lung? how is it easily distinguished? 4. What about the above tumors causes both to mimic lymphoma? |
1. spindle or epithelioid + S100, lack CD1a, CD21, CD35, B- and T-cell markers and keratin 2. Romanowsky b/c of the characteritic open chromatin, nucleoli and cytoplasmic granules w/ granulocytes and eosinophilic forms accompanying 3. Merkel cell carcinoma which is CK20 + 4. scant cytoplasm and necrosis that mimics lymphoglandular bodies |
|
|
|
1. What is a rule of metastases regarding supraclavicular nodes? 2. What cytomorphology suggests large cell carcinomas versus lymphoma? be site specific when possible |
1. Left: mets from below the diaphragm right: mets from above the diaphragm 2. predominance of clusters no lymphoglandular bodies abundant necrosis (colorectal and lung) signet ring cells (gastric and breast) abundant clear cytoplasm (RCC and ovarian) intranuclear inclusions (PTC and melanoma) |
|
|
|
1. Nasopharyngeal carcinoma (NPC) commonly manifests as cervical LAD similar to lymphoma, what is the cytomorphology? 2. What is the immunoprofile of IPC? 3. What other occult tumor often presents as cervical LAD? How can it be identified? |
1. clusters of undifferentiated large cells large nuclei with pale chromatin +/- prominent nucleoli moderate cytoplasm intermingled lymphocytes lymphoglandular bodies!!! 2. CK+ and EBV DNA or RNA CD45- 3. HPV driven SQC of the base of tongue, tonsil or oropharynx; + for CK, p16 and HPV ISH |
|
|
|
1.What is the cytomorphology of metastatic melanoma "the great masquerader"? 2. To where do testicular and mediastinal seminomas metastatsize? |
1. dispersed single cells and loose clusters epithelioid, spindle, and/or pleomorphic shapes eccentric nuclei binucleated (mirror image) forms nuclear inclusions single small to large nucleoli cytoplasm +/- melanin (50%) and vacuoles (best on air dried) NO lymphoglandular bodies 2. deep nodes of abdomen and chest, respectively |
|
|
|
1. What is the cytomorphology of seminomas? 2. Describe the characteristic background. 3. What other tumors display the above? |
1. dispersed large cells and focal clustering macronucleolus voluminous cytoplasm with peripheral, large, blisterlike vacuoles small round lyphocytes lymphoglandular bodies granulomas tigroid background 2. reticular or linear network of cytoplasmic strands and proteinaceous fluid 3. clear cell carcinomas |
|
|
|
1. How commonly do sarcomas metastasize to lymph nodes? 2. What 6 sarcomas do not hold true to the above? |
1. NOT <3% of patients with sarcomas have lymph node involvement 2. synovial sarcoma epithelioid sarcoma andiosarcoma rhandomyosarcoma kaposi sarcoma follicular dendritic cell sarcoma |
|
|
|
1. What is the cytomorphology of synovial sarcoma? 2. What are the two forms of kaposi sarcoma and what stains are needed to make the diagnosis? 3. What is the patient population of follicular dendritic cell sarcoma? IHC? |
1. large loosely cohseive syncitia of small, monomorphic bland cells with ovoid nuclei and find chromatin, smooth outlines and scant tapering cytoplasm; rarely acinar structures representing the epithelial component are seen 2. endemic and sporadic (associated with immunodificiency like AIDS or renal transplant); vascular makers CD31/34 AND HHV8 3. young to middle age adults; + CD21/23/35 and -CD45 |
|

