![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
Rate of reaction |

The change in concentration of reactants or products in a given time |
|
|
Collision frequency |
The number of collisions between particles per unit time in a system |
|
|
Activation energy |
The minimum energy the colliding particles need in order to react |
|
|
Pressure |
Less space for particles to move in More likely to collide |
|
|
Temperature |
More particles have activation energy- more kinetic energy More successful collisions |
|
|
Catalysts |
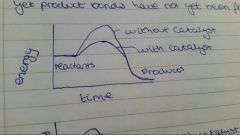
Provide alternative reaction pathway Lower activation energy- more particles have it More successful collisions |
|
|
Collision theory |
Particles must collide Sufficient energy Correct orientation |
|
|
Concentration |
Increases rate of reaction More particles in the area Collisions more likely |
|
|
Maxwell-Boltzman distribution |

|

