![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
If the overall order of a reaction is 0, what is the unit for the rate constant? |
M S^-1 |
|
|
If the overall order of the reaction is first order, what are the units for the rate constant? |
S^-1 |
|
|
If the overall order of the reaction is second order, what are the units for the rate constant? |
M^-1 S^-1 |
|
|
If the overall order of the reaction is third order, what are the units for the rate constant? |
M^-2 S^-1 |
|
|
What is the rate expression? |
-change in concentration/ change in time 🔺[ ]/🔺time |
|
|
What's the average rate of reaction? |
The rate at which a reaction proceeds over a time period. |
|
|
What is instantaneous rate? |
The rate at which a reaction is proceeding at a specific time or [ ]. |
|
|
What is the initial reaction rate? |
The instantaneous reaction rate at time zero. |
|
|
Given: aA + bB ➡️ cC + dD Relate the rates of consumption of the products to the rates of formation of the products. |
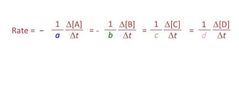
|
|

|
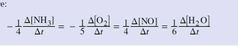
|
|
|
How does concentration affect the rate of a reaction? |
Higher concentration= faster rate |
|
|
How does temperature affect the rate of a reaction? |
Temperature increase=rate increase and vice versa |
|
|
For the reaction: aA + bB ➡️ cC + dD What is the rate law? |
Rate=k[A]^x[B]^y |
|

What is the overall order?
|

|
|
|
What does molecularity mean? |
The number of reactant species. |
|
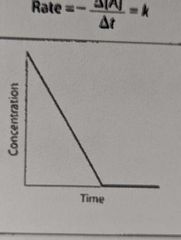
What is the overall order of this reaction? |
0 order |
|
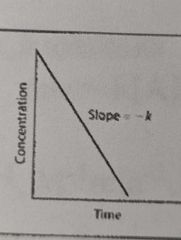
What is the overall order of the reaction? |
0 order |
|
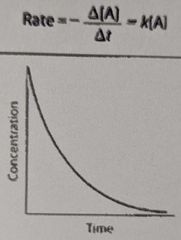
What is the overall order of the reaction? |
1st order |
|
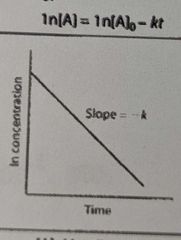
What is the overall order of the reaction? |
1st order |
|
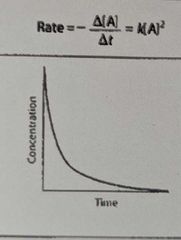
What is the overall order of the reaction? |
2nd order |
|
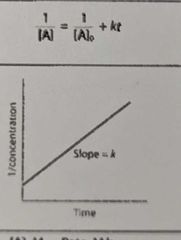
What is the overall order of the reaction? |
2nd order |
|
|
What are the 3 postulates of collision theory? |
1. The rate of the reaction is proportional to the rate of reactant collisions. 2. Must collide in correct/same orientation for bonding. 3. Collision must occur w/enough energy |
|
|
What is the Arrhenius equation? |
k=Ae ^-Ea/RT Where R=8.314J/mol/K and T=kelvin (c+273) |

