![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
What must particles do in order to react |
The particles have to collide with sufficient energy (activation energy) and the correct orientation |
|
|
Define Activation energy |
The minimum energy that particles must collide with for a reaction to occur |
|
|
Draw and label a Maxwell-Boltzmann Curve. |
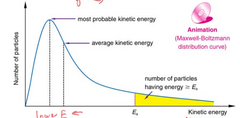
|
|
|
What happens to the rate of reaction when temperature is increased and explain why |
Increase in temperature leads to a higher rate of reaction. A larger portion of the particles in the reaction will have more energy than the activation energy. Therefore, there will be more successful collisions per second leading to an increased rate. |
|
|
What happens to the rate of reaction when the concentration/pressure is increased and explain why |
An increase in concentration/pressure will increase the rate of reaction. By increasing the pressure/concentration the number of particles in a given volume will increase. This leads to more frequent successful collisions. |
|
|
Define catalyst |
A substance which increases the rate of reaction by decreasing the activation energy and isn't used up in the reaction |
|
|
How do catalysts work and how do they increase the rate of reaction |
They provide an alternative pathway which has a lower activation energy. The path with a lower activation energy will increase the rate of reaction as the number of particles that have sufficient energy will increase. Therefore, there will be more frequent successful collisions, so increased rate of reaction |

