![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
64 Cards in this Set
- Front
- Back
|
What is the nicotinic, cholinergic receptor?
|
A ligand gated cation channel that antiports Na and K
|
|
|
What activates the nicotinic, cholinergic receptor?
|
ACh
|
|
|
What does nicotinic, cholinergic receptor activation produce?
|
depolarizing post-synaptic potentials.
*aka EPSPs. |
|
|
What receptor is the site of action of nicotine?
|
Nicotinic, cholinergic receptor
|
|
|
What happens when ACh is released by a motor neuron and it binds to a nicotinic, cholinergic receptor?
|
The receptor depolarizes skeletal muscle
|
|
|
What is myasthenia gravis?
|
The immune system makes Abs to the skeletal muscle nicotinic receptor resulting in muscle weakness.
|
|
|
What is the 5-Ht3 ionotropic receptor?
|
A ligand-gated cation channel that is selective for Na and K
|
|
|
What is the GABA-A ionotropic receptor?
|
A ligand-gated anionic channel that conducts Cl- current.
|
|
|
What does GABA-A activation produce?
|
Cl- selective anion channel that when activated by GABA mediates hyperpolarizing inhibitory postsynaptic potentials (IPSPs).
|
|
|
What do benzodiazepines and barbiturates do?
|
Act on the GABA-A receptor to make you sleepy!
|
|
|
What is the pentameric glycine ionotropic receptor?
|
A ligand-gated Cl anion channel that is activated by binding of glycine inhibitory IPSPs.
|
|
|
What is the function of the pentameric glycine ionotropic receptor?
|
Modulates excitatory neurons that mediate wake state and response to auditory and tactile stimuli.
|
|
|
What is startle disease?
|
"hyperplexia". Characterized by an abnormal response to auditory or tactile stimuli.
|
|
|
What is the glutamate-activated cation channel?
|
A ligand-gated channel that conducts Na and K that is activated by glutamate.
|
|
|
What does activation of the glutamate-activated cation channel produce?
|
EPSP and it is involved in long-term potentiation of memory.
|
|
|
What channel is involved in Rasmussens encephalitis?
|
NMDA (glutamate receptor)
|
|
|
What is the purinergic ligand-gated cation channel?
|
1. A post-synaptic ATP-activated cation channel that is permeable to Na, K and Calcium
2. involved in excitatory synaptic transmission and regulation of blood clotting. |
|
|
What is the role of ATP in purinergic ligand-gated cation channels?
|
Channels activated by synaptic co-release of ATP in catecholamine (NE) containing synaptic vesicles.
ATP-Ca channels are synergistic w/ alpha1 receptors and enhance NE mediated constriction. |
|
|
What is the cystic-fibrosis transmembrane receptor? (CFTR)
|
A ligand-gated channel that contains two internally homologous domains. It is a Cl- selective channel coupled to cAMP regulation.
*IMPORTANT transport pathway in secretory and absorptive epithelia in lung tissues. There are numerous mutations in this channel, one of which causes cystic fibrosis |
|
|
What is the CIC Cl- channel?
|
Cl- selective voltage-sensitive anion channel in skeletal muscle.
It regulates the regulation of electrical excitability in skeletal muscle. Modulates the threshold potential for activation in skeletal muscle. ALSO mediates Cl- transport in epithelia |
|
|
What mutation causes becker and thompson myotonia? what marks becker and thompson myotonia?
|
CIC Cl- channel; hyperexcitability of skeletal muscle causing delayed relaxation
|
|
|
What is the tetrameric intracellular IP3 activated channel?
|
VERY IMPORTANT CHANNEL IN SMOOTH MUSCLE!!!
1. intracellular cation channel permeable to Na, K and CALCIUM 2. Activated by binding of IP3 and Ca 3. mediates excitation-contraction in smooth muscle and participates in intracellular Calcium release. |
|
|
What is the mechanism of action of the CIC Cl- channel?
|
In smooth muscle ER, IP3 channel activation is coupled to alpha1-b receptor activation by NE, which in turn activates phospholipase C. PLC promotes the release of phospho-inositol bis phosphate (PIP2) w/ final conversion to IP3.
|
|
|
What is the function of the tetrameric intracellular IP3 channel?
|
Mediates excitation-contraction coupling in smooth muscle and participates in intracellular Ca release.
|
|
|
What is the function of the tetrameric ryanodine receptor (RYR) Ca release channel?
|
aka: CICR
Promotes rapid intracellular Ca release. |
|
|
What is the RYR1 channel activated by? where is it located?
|
Direct mechanical coupling to Cav channels in skeletal muscle.
located in skeletal muscle |
|
|
What activates RYR2 and RYR3 channels? where is it located?
|
Activated by elevation of cytoplasmic Ca due to plasma membrane Ca entry.
Located in heart, smooth muscle and neurons |
|
|
What is dysregulation of the RYR3 receptor linked to? what drug is more selective for the RYR3 channel?
|
malignant hyperthermia.
Dantrolene, an antagonist drug |
|
|
What is the multi-meric ORAI store operated Ca channel?
|
Located on the plasma membrane on non-excitable cells such as epithelial cells and lymphocytes.
*basically it is a plasma membrane Calcium channel. Orai is activated by IP3-activated Ca release and CICR from SER |
|
|
What is STIM?
|
An SER "calcium sensor" and is activated when SER Ca levels are lowered.
|
|
|
What is another name for the ORAI channel?
|
I-CRAC for Ca-release activated Ca current or SOC channel for store-operated Ca entry.
|
|
|
What is the essential function of a channel?
|
To form a aqueous channel pore so that ions flow across the hydrophobic membrane bilayer down the electrochemical gradient.
|
|
|
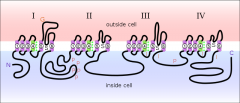
What is the structure of most eukaryotic channels?
|
Aqueous pores are located at the center of an oligomeric rosette-like arrangement of homologous subunits.
Ea. subunit is a polypeptide that weaves through the membrane several times |
|
|
What are gap junctions?
|
Protein channels that connect two cells w/ a large, unselective pore that allows ions and small molecules as large as 1 kDa to pass through.
|
|
|
What is a connexin?
|
A subunit that is an integral membrane protein that, (along w/ 5 other subunits) forms a gap junction.
Each subunit has four identifiable hydrophobic transmembrane segments |
|
|
What effect does intracellular Ca concentration have on the probability a gap junction will be open/closed?
|
*elevated [Ca] increase the probability that the gap junction will be closed.
|
|
|
Where are the N1 AChRs located?
|
At the neuromuscular junction
|
|
|
Where are the N2 AChRs located?
|
In the ANS on the postsynpatic membrane of the postganglionic sympathetic and parasympathetic neurons and in the CNS
|
|
|
what change in the nicotinic receptor occurs during development?
|
In fetal skeletal muscle the subunit composition is alpha2-beta-gamma-delta but in the adult there is a transition from gamma to epsilon, so the adult nicotinic receptor is alpha2-beta-epsilon-delta
|
|
|
How many subunits does the nicotinic receptor have?
|
5. The ACh receptor shows pentameric radial symmetry that corresponds to a rosette.
|
|
|
How can ALL potassium channels be modulated?
|
By reversible phosphorylation
|
|
|
What is the structure of the Kv channel?
|
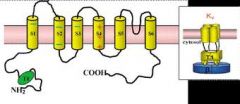
4 identical tetrameric subunits w/ six transmembrane regions on ea. subunit.
|
|
|
What is the structure of small and intermediate conductance calcium activated potassium Channels? (SKca and IKca)
|
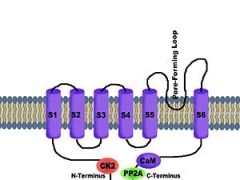
They have the same basic structure as the Kv channel, but these channels are voltage independent and only activated by Calcium.
|
|
|
What is the voltage sensitive region of the Kv channel?
|
region 4
|
|
|
What is the structure of the inward rectifier Potassium channel?
|
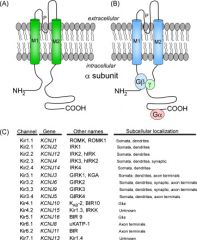
Kir is a tetramer w/ two membrane spanning regions.
|
|
|
What is the structure of the dimeric tandem two-pore potassium channel (K2P)?
|
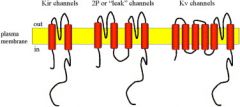
Basically a tandem duplication of a Kir channel
|
|
|
What is the hyperpolarization-activated, cyclic nucleotide-gated cation channel (HCN)?
|
Plays a CRITICAL role in the automaticity of the heart and rhythmically firing neurons of the brain.
|
|
|
What regulates HCN channels?
|
Cyclic nucleotides and they are also voltage sensitive.
|
|
|
What are CNG channels?
|
A family of cation-selective channels that are directly activated by intracellular cGMP or cAMP.
These channels play a IMPORTANT role in visual and olfactory sensation. |
|
|
What activates the CNG channel?
|
binding of cAMP or cGMP
|
|
|
What are the TRP channels?
|
Sensory channels that transduce sensory information
|
|
|
What activates the TRPV channel?
|
Capsaicin, the "hot" ingredient of chili peppers. Appears to function in pain and temperature sensation
|
|
|
What activates the TRPM channel?
|
Menthol, the "cool"-tasting substance in eucalyptus leaves.
|
|
|
What is the structure of the voltage-gated sodium channels? (Nav)
|
Four domains, each domain containing the S1 to S6 structural motif that is homologous to the Kv K+ channel monomers. Each domain has 1 voltage sensing region.
|
|
|
What unique structure are the Nav channels associated with?
|
A unique family of auxiliary beta-subunits, modifying gating behavior and membrane localization of the channel-forming alpha-subunit.
|
|
|
What do mutations in the Nav beta-subunit result in?
|
Altered Nav function which causes Long QT (LQT3) syndrome.
*LQT3 occurs at fast cardiac paces (i.e. exercise/stress) |
|
|
What is the structure of voltage-gated Calcium channels?
|
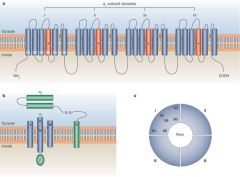
Cav channels are multisubunit complexes (similar to Nav) that are called the alpha-subunit. the alpha subunit has four voltage sensor regions and in addition there are three accessory proteins that are involved in channel regulation. (alpha2-delta and a gamma subunit)
|
|
|
What is the general structure of ligand-gated channels? What channels have this basic structure?
|
*basic structure: pentamers w/ 4 transmembrane regions.
*channels that follow this: ACh, 5-HT, GABA, Glycine |
|
|
What major structures are absent on ligand-gated channels?
|
Phosphorylation sights, so they are NOT regulated by reversible phosphorylation
|
|
|
What is the structure of the Glutamate channel?
|
ligand channels that have only three membrane spanning regions per monomer
|
|
|
What is the structure of the purinergic ligand-gated channels?
|
A pentameric dimer w/ two membrane spanning regions
|
|
|
What is the CFTR channel?
|
a Cl- channel that modulates fluid balance in the lung alveoli.
*cystic fibrosis is caused by mutations in the NBD1/NBD2 regions |
|
|
What is the structure of the CIC family of Cl- channels?
|
have FIFTEEN (15!!!) membrane spanning regions on EACH monomer
|
|
|
What is the structure of the ORAI channel and STIM?
|
![ORAI is a monomer until SER Ca levels decline.
The decline in SER [Ca] activates STIM aggregation.
STIM aggregates causing ORAI aggregation and formation of Ca channels.](https://images.cram.com/images/upload-flashcards/55/24/49/2552449_m.jpg)
ORAI is a monomer until SER Ca levels decline.
The decline in SER [Ca] activates STIM aggregation. STIM aggregates causing ORAI aggregation and formation of Ca channels. |

