![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
What is the difference between T cell independent and dependent antigens?
|
T CELL INDEPENDENT ANTIGENS
-T cells recognize peptides bound to MHC molecules -therefore, antigens that possess no peptide structure cannot be recognized by T cells -these antigens are called thymus-independent antigens and include lipopolysaccharide from the cell envelope of gram negative bacteria and polysaccharide capuslar antigets -these antigens may directly stimulate B cell to cause proliferation and secretion or antibody, or they may act as B cell mitogens, directly causing mitosis regardless of the cell's antigenic specificity -the response to thymus independent antigens is generally weaker than the response to other classes of antigens, resulting in the secretion of IgM antibodies only and the absence of immunologic memory T CELL DEPENDENT ANTIGENS -most antigens introduced into the body fall into the category of thymus-dependent antigens -response to such molecules requires the direct contact of B cell with TH cells and their cytokines |
|
|
What is the B cell and T cell collaboration paradox?
|
-T cells and B cells recognize antigen in very different forms
-B cells recognize epitopes that are conformational and relate to the physiochemical properties of the antigen surface -T helper cells provide help only to those B cells that are recognizing the same antigen |
|
|
How do T cells help B cells?
|
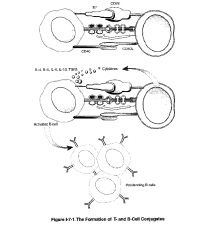
-T cells are activated when the T cell receptor recognizes the MHC molecule loaded with a foreign peptide
-these T helper cells expand in a clonal way and form memory T cells -B cells that are specific for the antigen bind to the antigen to their surface Ig -the binding allows the B cell to take up the antigen and process it in the MHC II pathway and present it on the surface of the B cell -the peptides are presented are the same one to which the T cell has been previously primed because the antigen processing pathway is the same and the MHC II is the same as the one used to expand the T cell initially -T cells helping B cells involve close contact between T cells and B cells -this is necessary because the T cell receptor needs to interact with the peptide loaded MHC II molecule on the surface of the B cell -while B cells can present antigen to memory T helper cells, they cannot activate naive T cells that have not yet been activated -activation of naive T cells requires dendritic cells -after the MHC II/peptide conjugate is inserted into the membrane there is expression of costimulatory molecules such as B7 on the B lymphocyte, making them effective presenters of antigen to TH cells in the area -once the TH cell recognizes the processed antigenic peptide on MHC II the 2 cells form a conjugate, and the TH cell is activated and induced to become a TH 2 cell -TH 2 cells in conjugates rearrange their golgi apparatus toward the junction with the B cell leading to the directional release of cytokines toward the B cell -in addition expression of a molecule known as CD40 on the B cell provides the second signal for B cell activation -the B cells are encouraged to proliferate after this interaction -the final signal delivered by the TH2 cell is the release of cytokines, which induce the differentiation of B cells into fully differentiated, antibody-secreting cells and memory cells and induce class switching |
|
|
What is affinity maturation and what role do T cells play?
|
-B cells undergo hypermutation in variable parts of their surface Ig
-some of these mutations will increase the affinity to the antigen while others will decrease the affinity to the antigen -thus B cells have to compete for antigen -those B cells with an increased affinity for the antigen will be able to continue to take up and present the antigen to the T cells, while there will be a stage when the low affinity B cells will not be able to effectively take up and present antigen to the T cell -these B cells that cannot compete for the antigen will die from apoptosis because they lack T cell help -the high affinity B cells will continue to receive T cell help and will be activated to form plasma and memory B cells -Thus over time the affinity of the antibodies secreted by plasma B cells will increase and the affinity of the Ig on the surface of memory B cells is generally higher than that of the initial population of B cells |
|
|
What does cell mediated immunity include?
|
●CD4+ T helper cells help other cells get activated (TH1 helper of macrophages + IgG2 production and TH2 helper of B cells produce antibodies)
●CD8 T cytotoxic cells kill infected cells ●T regulators are suppressor T cells involved in down-regulating immunity for example against self antigens ●γδ T cells are primitive T cells with a limited T cell repetoire |
|
|
What are the 3 signals needed to activate T cells?
|
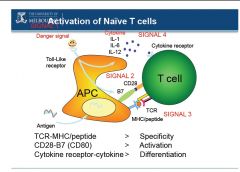
●Signal 1
-antigen recognition through binding of T cell receptor to the MHC peptide complex -this gives specificity to the T cells -the CD4 or CD8 co-receptors bind to the constant part of the MHC complexes and help in this process ●Signal 2 -co-stimulatory signal essential to activate naive T cells (not required for memory T cells) -for example B7 on T cell binds CD28 on antigen presenting cells (APCs) -this is why naive T cells can only be activated by dendritic cells -the co-stimulatory signal on the APC is often present only after pathogen specific "danger signals" are detected by the APC ●Signal 3 -cytokines present in the environment where interaction between T cell and APC occurs -this allows cells to proliferate (for example through IL-2) and differentiate (for example IL-12 for TH1 and IL-4 for TH2) |
|
|
What is the function of cytotoxic T cells?
|
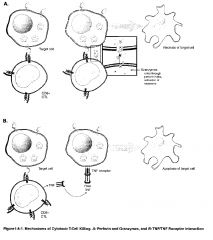
-the CTL recognizes the cell it will ultimately kill by interaction between its TCR and MHC class I antigens on the surface of the target cell
-if the cell in question is performing normal functions and therefore producing normal "self" peptides, there should be no CD8+ T cells that have a complementary TCR structure -if the cell is infected with an intracellular parasite or is expressing neoantigens reflective of tumor transformation, however, some small proportion of those CD8+ cells generated from the thymus should be capable of binding their TCRs to this MHC class I/non-self peptide combination -CTLs are capable of differentiation and cloning by themselves in the presence of appropriate non-self peptide/class I MHC antigen stimulus, but are much more effective in doing so if they are assisted by signals from TH1 cells -the TH1 cell secretes IL-2 which acts on CD8+ cells to enhance their differentiation and cloning -interferons poduced in the area will increase the expression of MHC molecules to make targets more susceptible to killing -CTLs kill their target by delivery of toxic granule contents that induce apoptosis of the cell to which they attach -this process occurs in 4 phases: 1) attachment to the target (mediated by TCR, CD8, LFA-1 integrin) 2) Activation (cytoskeletal rearrangements to concentrate granules against attached target) 3) Exocytosis of granule contents (perforin & granzymes) 4) Detachment from the target -the death of the target may be mediated in distinct fashions -first, perforin present in the CTL granules creates pores in the membrane of the target cell through which granzymes (serine proteases) enter the target, inducing the activation of caspases which activate the "death domain" -second, cytokines such as IFN-γ with TNF-α or TNF-β can induce apoptosis -furthermore, activated CTLs express a membrane protein called Fas ligand (FasL), which may bind to its complementary structure on the target, Fas -when this occurs, caspases are induced and death results |
|
|
How are TH1 & TH2 subsets produced?
|
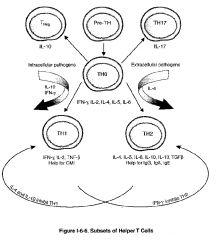
-there are 2 major and 2 minor classes of helper T cells (TH) cells, both of which arise from the same precursor, the naive TH lymphocyte, sometimes called the TH0 cell
-differentiation of a TH0 cell into a TH1 cell seems to be stimulated by microbes that stimulate a strong initial innate immune response with the resultant production of IL-12 by macrophages or IFN-γ by NK cells -the differentiation of TH1 cells is stimulated by many intracellular bacteria -differentiation of a TH0 cell into a TH2 cell seems to be encouraged in the absence of such innate immune stimuli, perhaps by default when persistent antigen remains in the system without significant macrophage of NK stimulation -in this way, naive TH0 cells seem to produce IL-4 constitutively, and in the absence of IL-12 stimulation, these cells will upregulate their production of IL-4 to encourage differentiation into TH2 cells -the differentiation of TH2 seems to be favored in infections with helminths and in response to allergen |
|
|
What are the effector mechanisms of TH1 cells?
|
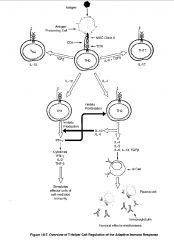
❤Activate macrophages to destroy intracellular microorganisms more efficiently through soluble IFN-γ and membrane bound CD40 ligand (binding of CD40 on macrophages)
-activated macrophages become highly microbiocidal by increasing expression of MHC molecules, B7 molecules, TNF receptors, nitric oxide, proteases & oxygen radicals -thus TH1 cells through macrophage activation play a critical role in host defense against intracellular and engulfed extracellular pathogens e.g. mycobacteria tuberculosis or leprae that resist killing in non-activated macrophages ❤Induce apoptosis of infected macrophages ❤release cell recruitment & chemotactic factors (MCF) and induce macrophage differentiation in bone marrow (IL-3, GM-CSF) ❤Activation of B cells to produce opsonising antibodies EFFECTOR MOLECULES: ❤TH1 cell cytokines: IFN-γ, TGF-β, and IL-2 (also GM-CSF) -IFN-γ produced by TH1 inhibits TH2 ❤Membrane bound CD40 ligand & Fas ligand -Fas ligand binds to FAS on infected macrophages and hence induce apoptosis of infected macrophages |
|
|
What are the TH2 effector mechanisms?
|
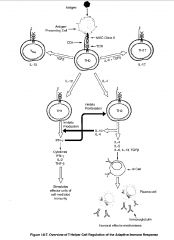
-specialized for B cell activation and drive B cells to proliferate and differentiate and produce neutralizing Ab
Functions: ❤Initiation B cell response by activating naive B cells to proliferate & secrete IgM ❤Activating B cells to proliferate, differentiate, and produce other neutralizing Ab eg. IgG, IgA, IgE -often produce Ab that can bind toxins and fix complement ❤Involved in mast cell and eosinophil growth & development EFFECTOR MOLECULES: ❤Cytokines: IL-4, IL-5, IL-6, IL-10, IL-13, and TGF-β -IL-4 ad IL-10 produced by TH2 inhibit TH1 ❤Membrane bound CD40 ligand that binds to CD40 on B cells and induce B cell proliferation is an important second signal for naive B cells -inefficient at activating macrophages because IL-10 is inhibitory for macrophages & they produce little IFN-γ |
|
|
What are T Reg cells & what types do we have?
|
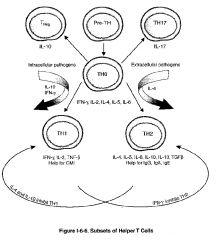
-a third population of T cells which arises from the TH0 is called the Treg (T regulatory cell)
-these cells regulate (inhibit) T cell function -they are identified by their constitutive expression of CD25 on their surface and by expression of the transcription factor FoxP3 -they secrete inhibiting cytokines such as IL-10 and have been shown to be critical for the prevention of autoimmunity -there are natural Tregs: CD4+CD25+ T cells which develop, and emigrate from the thymus to perform their key role in immune homeostasis -adaptive Tregs: are non-regulatory CD4+ T cells which acquire CD25 expression outside the thymus, and are typically induced by inflammation and disease processes, such as autoimmunity and cancer |
|
|
What are TH17 cells and what types do we have?
|
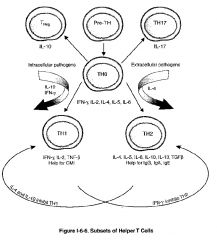
-the final population of TH cells which arises from TH0 is called the TH17 cell
-these cells are identified by the expression of transcription factor RORγt (retinoic acid receptor-related orphan receptor-γt) and by their production of the proinflammatory cytokine IL-17 -they are believed to play a role in the tissue damage associated with some autoimmune diseases |

