![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
Ethene |
Hydrocarbon has a double Bond (Alkenes) |
|
|
Eth- |
2 Carbon Atoms |
|
|
Oct- |
8 Carbon Atoms |
|
|
Making Alkenes into Polymers |
This is called Polymerisation and this is when lots of small alkenes are made into one large polymer wich are joined up as a chain. |
|
|
Meth- |
1 Carbon Atom |
|
|
Prop- |
3 Carbon Atoms |
|
|
But- |
4 Carbon Atoms |
|
|
-Ane |
Hydrogen Atom on all Carbon Atoms |
|
|
What does a Polymer Look like? |
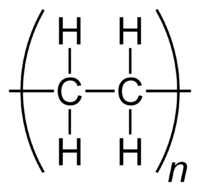
|
|
|
Equation for an Alkane |
Cn+H2n+2 |
|
|
Equation for an Alkane |
Cn+H2n |
|
|
Carboxylic Acids |
Double Bond Between Carbon and Oxygen Single Bonds between OH and any Hydrogen Atoms |
|
|
Alcohols |
Carbon atoms surrounded by Hydrogen Atoms and always has single bond of Oxygen followed by another Hyrogen. |
|
|
Formation of Esters |
Formed from an alcohol and a carboxylic acid |
|
|
Equation for Ester |
Alcohol + Carboxylic Acid -> Ester + Water |
|
|
-oate |
Esters |
|
|
Uses for Esters |
*Perfumes (smell nice) *Flavourings and Aromas Solvents for Paint and Nail Varnish,etc. |
|
|
Impacts of Polymers |
*Not Biodegradable - Causes Problems with Litter -Hard to Dispose of -Can only be recycled a few times before they degrade too much. |

