![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
208 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Describe the steps of platelet plug formation
|
adhesion: integrins on platelet membrane bind to exposed collegen
activation: secretion of ADP and thromboxane A2, formation of spiky proccesses protoming adhesion aggregation: +ve feedback by ADP causes other platelets to become activated. Surrounding tissue is protected by secreting NO and prostacyclin Actin-myosin tightens and strengthens platelet plug Vasoconstictors secreted: thromboxin A, seritonin, adrenaline |
|
|
|
When blood vessels
are damaged, how are blood clots formed |
Extrinsic (tissue factor) pathway: factor VII leaves vasculature and contacts interstitial tissue factor
The TF-VIIa complex activates IX and X Prothrombin becomes thrombin Thrombin converts fibrinogen to fibrin |
|
|
|
Describe the contact activation pathway of coagulation
|
Factor XII binds to collegen with other factors
Converts to XIIa which converts XI to XIa which converts IX then the common pathway to thrombin and fibrin |
|
|
|
How are clots normally dissolved
|
Plasminogen becomes plasmin by factor XIIa, XIa.
Also tissue plasminogen activator from endothelial cells prduces plasmin to lyse clots |
|
|
|
List the genetic requirements needed to develop cancer
|
1) initiation: initial DNA damage leading to loss of control of the cell cycle and genetic instability
2) progression: repetition of the cell cycle with genetic instability resulting in multiple mutations |
|
|
|
List the 4 stages of cancer development
|
Dysplasia: changes in cellular morphology (dysplasia, neoplasia) and tumour formation
Carcinoma in situ: immature cells unable to invade basement membrane Invasive carcinoma: development of proteolytics and motility to invade tissue 4) metastasis: ability to survive in the bloodstream, extravagate, invade other tissues, proliferate and angiogenesis |
|
|
|
Using the example of the Rb tumour supresor gene, why are some people more susceptible to tumour formation
|
Rb is dominant, therefore mutations act recessively
If an indicidual inherits only one functioning Rb gene then they are likely to form tumours |
|
|
|
Pick the correct answer:
a) tumour supressor gene mutations act dominantly whereas oncogenes act recessively b) tumour supressor gene mutations act recessively whereas oncogenes act dominantly |
b)
|
|
|
|
What are the 3 ways oncogenes are produced
|
1) point mutation
2) gene amplification 3) chromosomal rearrangement |
|
|
|
What type of gene is the Philadelphia chromosome, what is the condition that it causes and what is the mechanism
|
1) oncogene
2) CML 3) Translocation produces a bcr-abl hybrid tyrosine kinase transduction molecule allowing cell cycling independent of extracellular growth signals |
|
|
|
What 4 things would you ask in order to determine if a patient has malignant breast cancer
|
1) how long has the lump been (short is bad)
2) are there axillary lumps, enlarged abdomen (liver) 3) is there pain (malignant tumours are painful) 4) contraceptives? alcohol? children? 4) family Hx of BC |
|
|
|
How does the course of cancer lump growth determine its characteristics and aids during examination
|
Cancer: infiltrates and attaches to other types of tissue (muscle). Becomes fixed. Margin is irregular and spiculated
Benign: mobile with smooth margin, pushes away other tissue, does not adhere |
|
|
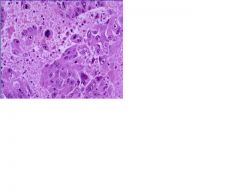
What are the distinctive features of cancer cells in biopsies
|
1) pleomorphism
2) necrosis due to overly rapid growth exceeding blood supply 3) darkly staining nuclei 4) loss of polarity 5) mitotic bodies |
|
|
|
What are the 4 stages of pathological examination of cancerous tissue
|
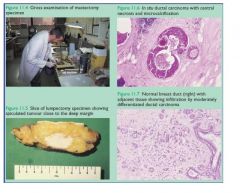
1) Gross morphology of overall tissue
2) gross examination of sliced tunour looking for smoothness or irregular/spiculated border 3) low power looking for areas of necrosis, calcification, dark staining 4) High power looking for abnormal cells: pleomorphic, dark staining, loss of polarity (differentiation) |
|
|
|
A pathological lump is removed from an organ and examined histologically. What would indicate whether it is a primary or secondary
|
Primary: presence of dysplasia (if full thickness it is in-situ carcinoma) adjacent to the metastatic carcinoma shows the evolution of the tumour
|
|
|
|
A section of bowel showed adenomas in the mucosa. Histology revealed irregular spiculated masses infiltrating the muscularis. what can be said about this investigation?
|
Presence of dysplasia (adenoma) indicated that it is a primary cancer. Irregularly shaped lesion indicated cancer. Invasion of muscle and multiple tumours indicate metastasis (one tumour would indicate more likely an in-situ carcinoma).
|
|
|
|
What is the cancer grading scale of breast cancer based on
|
1) pleomorphism
2) mitotic activity 3) degree of tubular formation (ie differentiation) |
|
|
|
What is the common cellular process occuring during the proliferative stage of the menstrual cycle and lactation
|
Physiological (normal) Hyperplasia - increase in cell number
|
|
|
|
What cell process goes on in the liver when a person shows ascites, portal hypertensiion and varices
|
Pathological hyperplasia of hepatocytes and fibroblasts leading to replacement of normal structure and fuction with connective tissue
|
|
|
|
What would you expect to find when palpating the hypogastric region where a patient has (a) carcinoma in-situ of the prostate and (b) BPH
|
a) tumour likely to be in peripheral zone, hence no bladder hypertrophy
b) in BPH, pathological hypertrophy of the tranzitiional zone leads to ureter blockage leading to bladder smooth muscle hypertrophy and palpable bladder |
|
|
|
What types of atrophy are the following:
a) shrinkage of the thymus gland with age b) changes to arm muscle mass mass in a cervical vertebral ventral ramus lesion |
a) physiological atrophy
b) pathological atrophy of the arm muscles |
|
|
|
How is it possible for adenoma to be formed in the epithelium of the oesophagus
|
Adenoma = glandular origin
With Barrett's oesophagus, mucous secreting glandular epithelium from the stomach replaces squamous epithelium, hence glandular adenoma forms |
|
|
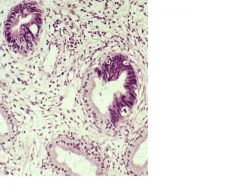
What is happening here in the cervix
|
Cell of the gland have undergone pre-cancerous dysplasia: large nuclei showing abnormal DNA, mitotic activity and disorganisation of the smooth glandular epithelial structure
|
|
|
|
How is neoplasia different to hyperplasia or metaplasia
|
Both of these are responses to external stimuli, are reversible and the cells still have normal control over the cell cycle - hyperplasia: proliferation of same cell type
metaplasia: proliferation of a different cell type In neoplasia: the cell cycle is irreversible and cells can proliferate without external growth factors (loss of control of the cell cycle) |
|
|
|
What is the difference between ectopic tissue and a hamartoma
|
1) ectopic: normally organised tissue in the wrong place
2) hamartoma: correct types of tissue in the right place (eg stroma and ductal tissue of the breast) but abnormally organised resulting in loss of architecture and function |
|
|
|
With cellular hypoxia, what processes occur
|
ATP reserves frop to 5-10%, Na/K pump ceases, intracellular sodium and calcium rises, extracellular potassium rises, abnormal protein folding and enzyme function disables apoptotic pathways -> necrosis
|
|
|
|
Describe the type of cellular injuty that occurs in hepatocytes following acute alcohol intoxication
|
steatosis: reversible cell injury caused by deposition of fat within the cell and hypertrophy
steatohepatitis: inflammation and necrosis but maintenance of lobular structures cirrhosis: chronic hepatocyte damage activates space-of-Disse stellate cells causng collagen deposition, activation of NKT cells, constriction and thickening of sinusoid walls preventing normal liver function (indicated by liver function tests: albumin and prothrombin) |
|
|
|
What are the histological changes evident under the microscope of necrotic cells
|
Nuclear shrinkage or disappearance (karryorhexis, karryolysis, pyknosis)
Eosinophilia as RNA is depleted and proteins denature Moth eaten cytoplasm Glassy cytoplasm as glycogen diappears myelin figures (structures forms from phospholipids breaking off membranes and spontaneously reforming) |
|
|
|
List the 5 types of necrosis and a typical tissue where it occurs
|
coagulative (myocardium)
caseous enzymatic fat necrosis (liver) liquifactive (brain) gangrenous |
|
|
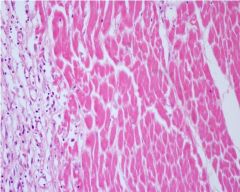
In this myocardium, decribe what has occurred and reasons for your conclusions
|
coagulative necrosis following MI
1) cells lack nuclei but structure is maintained 2) eosinophilic indicating loss of RNA and denaturing of proteins |
|
|
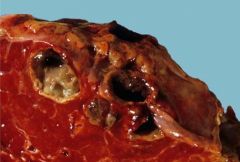
Describe what has happened to the cells within this lung lesion and why
|
Liquifactive necrosis within a staphylococcal abcess due to infiltration of immune cells releasing proteolytics and breaking down the connective tissue framework that would have preserved the cell shapes in coagulative necrosis
|
|
|
|
Describe fat necrosis
|
Pancreatitis causes release of lipases breaking down TAGs within adipocytes in the peritoneum causing their necrosis, releasing FFAs. FFAs combine with the released calcium stores forming interstitial chalk (calcified deposits)
|
|
|
|
What are the distinctive morphological features of cells undergoing apoptosis
|
1) Cells become crescent shape and form membrane bound blebs on surface
2) nucleus condenses and can fragment 3) Cells breakdown into small membrane bound apoptotic bodies containing protein, organelles and chromatin |
|
|
|
What is the difference between HbA and HbA2
|
HbA - adult haemoglobin (2 alpha and 2 beta subunits)
HbA2 - adult A2 haemoglobin (2 alpha and 2 delta subunits) |
|
|
|
What is the transferrin level in iron overload and why
|
transferrin synthesis increases with decreasing iron level. also Increased uptake of transferrin by cells (erythrocyte precursors etc)
|
|
|
|
List 4 factors that will shift the O2/haemoglobin curve to the right
|
Increased:
CO2 Acidity temperature BPG |
|
|
|
Explain the mechanism of how 2,3 BPG affects the O2/Haemoglobin dissociation curve
|
1) Unbound is in R (relaxed) state and high affinity for O2
2) 2,3 BPG binds to central site forcing T (taught) state (low affinity) 3) O2 dissociates |
|
|
|
What is the fraction of foetal haemoglobin at birth
|
50%
|
|
|
|
How does Hbf affect the O2/haemoglobin dissociation curve of the foetus. explain
|
Hbf has low affinity for 2,3DPG and remains in the taught form, shifting the haemoglobin curve to the left.
|
|
|
|
What are the 2 ways in which gene mutations affect protein function
|
1) Protein quality: eg sickle cell
2) protein quantity: eg thalassaemia |
|
|
|
Explain the effects on organs as a consequence of thalassaemia
|
1) bone thinning and expansion due to increased erythropoeiesis
2) spleen enlargement due to increased reabsorption of RBCs and haemolysis 3) tissue anoxia due to hypochromic, microcyticerythrocytes 4) hepatosplenomegaly due to extramedullary haematopoiesis |
|
|
|
A patient is diagnosed with sickle cell anaemia. Explain the signs found during physical examination
|
jaundice and splenomagaly due to high rate of haemolysis
|
|
|
|
List 3 ways in which anaemia can occur due to deficient production of RBCs
|
1) insufficient EPO
2) defective DNA production (folate, B12) causing apoptosis 3) iron deficiency |
|
|
|
List 3 ways in which anaemia can occur due to haemolysis
|
1) abnormal haemoglobin (thalassaemia, sickle cell anaemia)
2) membrane instability 3) metabolic disorder eg G6P ehydrogenase absence |
|
|
|
Define MCHC
|
Amount of haemoglobin in a volume of packed red blood cells ie haemoglobin (G/L) / haematocrit (% of 1L of blood volume)
|
|
|
|
Define MCV
|
volume of one red blood cell ie haematocrit (% of 1L of blood volume) / No of RBCs in a litre of blood
|
|
|
|
Explain macrocytic normochromic anaemia where MCHC is normal
|
1) B12 or folate deficiency results in inadequate uracil -> thymidine conversion, slowing DNA replication and cell cycle of reticulocytes
2) RNA generates globin at full speed resulting in large cells packed with normal concentration haemoglobin (macrocytic, normochromic and normal MCHC) |
|
|
|
Which transporter carries iron across the basolateral membrane of enterocytes
a) ferritin b) transferrin c) DMT1 d) ferroportin |
d) ferroportin
|
|
|
|
How is Fe3+ absorbed by enterocytes
|
1) fe3+ is reduced with vitamin C as a cofactor to Fe2+
2) Fe2+ is transported across apical membrane by DMT1 (and any other divalent metals) 3) Ferretin transports iron intracellularly to basolateral membrane 4) transportin delivers Fe2+ to transferrin, which binds to receptors on cells eg erythrocytes |
|
|
|
Patients with haemochromatosis exhibit mutations in the HFE gene. Explain the mechanism
|
HFE is an Fe regulatory protein binding to TfR, inhibiting uptake of transferrin-Fe and endocytosis once within cells, causing Tf-Fe to be ejected from the cell.
If HFE is defective, iron enters cells without regulation and accumulates within cells causing haemachromatosis |
|
|
|
How does the liver regulate the amount of iron aborbed by the gut
|
1) hepatocytes detect increased HFE
2) Releases hepcidin which binds to ferroportin, causing endocytosis and removal 3) enterocytes accumulate Fe and are sloughed off |
|
|
|
If you have blood group O-, what antibodies are present
|
anti A
anti B anti D (only after prior sensitization eg birth of Rh+ child without administration of anti-D antibody treatment) |
|
|
|
How does the breast remain mobile over its deep fascia
|
retromammary space
|
|
|
|
What makes up a breast lobule
|
1) alveoli
2) intralobular ducts |
|
|
|
A pregnant woman complains of small lumps around her nipple. What do you tell her
|
Montgomery glands - secrete sebum that prevents nipple cracking
|
|
|
|
What is the extent of the breast
|
1) horizontally: mid axillary line to the sternum
2) 2nd to 6th rib |
|
|
|
which quadrant is the longest part of the breast
|
upper outer quadrant - tail of Spence
|
|
|
|
What are the 4 areas of lymphatic drainage of the breast
|
1) axillary nodes
2) supraclavicular nodes 3) other breast 4) parasternal nodes |
|
|
|
What are the 3 main arteries supplying the breast
|
All originating ffrom the subclavian:
1) lateral thoracic 2) internal thoracic 3) thoracoacromial artery |
|
|
|
Describe the steps of platelet activation and inhibition
|
1) Normal endothelium releases NO and prostacyclin inhibiting platelet activation
2) Endothelial damage releases vWF and collagen activating platelets 3) Activated platelets secrete ADP, Ca++ and thromboxane A2 causing aggregation and platelet contraction |
|
|
|
Once platelets have been activated, describe he steps of thrombus formation
|
1) Foctor VII contacts tissue factor forming VII-TF complex causing 1st wave thrombin burst: Activation of IX and X -> prothrombin -> thrombin -> fibrinogen -> fibrin
2) 2nd wave: thrombin activates factors V and VIII 5) VIIIa combines with IXa to activate thrombin and continue coagulation 6) thrombin activates XIII to cause covalent linkages in fibrin, stabilising the thrombus |
|
|
|
Describe how a thrombus is localised and eventually broken down
|
1) Release of NO and prostacyclin from undamaged endothelium prevents thrombus spread to healthy endothelium
2) heparin like compound increases antithrombin III binds to IX, X, XI, XII inactivating them 3) thrombin activates protein-C degrades VIIIa and Va |
|
|
|
Describe the pathophysiology of BPH
|
1) Testosterone -> 5-alpha reductase (stromal cells) -> DHT (10x more potent than testosterone) causes hyperplasia of glandular and stromal cells, as well as hypertrophy of some stroma
2) Formation of discrete nodules in TZ increasing pressure on the prostatic urethra |
|
|
|
What would you ask regarding a patient with dysurea, polyurea, hesitancy and incomplete voiding
|
1) pain or cloudy discharge: UTI, infection
2) rate of onset of symptoms and other cancer related questions (weight loss, pain, past PSA results, DRE) - weeks may indicate malignancy, months may be BPH 3) history of diabetes - autonomic neuropathy, loss of bladder and sphincter control |
|
|
|
Where is BPH and prostatic cancer likely to be locatted within the prostate
|
BPH: TZ or middle lobe
Cancer: posterior zone or inferoposterior lobe |
|
|
|
What is the difference between pathological hyperplasia nad neoplasia
|
1) Hyperplasia: responsive to growth factors, therefore, removal of factors reverses the process
2) Neoplasia: irreversible cell replication due to deregulation of cell cycle |
|
|
|
Explain the difference between metaplasia and dysplasia
|
Metaplasia: replacement of one normal cell type with another one that is not normally found in that region
Dysplasia: abnormal changes to a cell due to DNA changes: 1) premalignant state and cells resemble malignant cell morphology 2) large irregular hyperchromic nuclei 3) reduced cytoplasm 4) large pleomorphic cells 5) loss of architecture and function |
|
|
|
Describe the different mutation types
|
1) Missense: substitution causing synonamous or non-synonamous mutation
2) nonsense: stop codon 3) frameshift: deletion or addition |
|
|
|
What is the likely consequence of a synonamous missense mutation
|
1) mRNA instability
2) decreased level of the protein 3) depletion of rarely used tRNA molecules causing lower overall protein levels |
|
|
|
True of false: OCD is related to a reduced number of dopamine receptors that is affected by:
1) a nonsense mutation 2) a missence non-synonamous mutation 3) a missence synonamous mutation |
1) false: would result in no receptors
2) true: could result in functioning but structurally unstable receptors decreasing their number on the cell surface 3) true: decreased mRNA stability and depletion of rare tRNA will reduce receptor expression |
|
|
|
Name a qualitative and quantitative globinopathy
|
1) qualitative: sickle-cell
2) quantitative: thallasaemia |
|
|
|
sickle cell anaemia is an autosomal recessive disease.
1) What would be the carrier rate and affected rate in offspring of an affected mother and carrier father 2) two carrier parents |
1) carrier, 50% afffected
2) carrier 50%, affected 25% |
|
|
|
Describe the main areas of the CNS responsible for analgesia
|
1) periaquaductal grey (PAG) sends central efferents and the NRPG sends feedback from the dorsal horn to the nucleus Raphe magnus
2) The NRM projects inhibitory fibres to the dorsal horn, releasing enkephalin |
|
|
|
Describe the neurons synapsing with dorsal horn fibers and how they modulate pain
|
1) c-fibers: unmyelinated, have pre-synaptic mu, delta and kappa receptors
2) descending fibers from the nucleus Raphe magnus secreting NA and 5HT 3) inhibitory interneurons secreting enkephalins, dynorphine |
|
|
|
What are the 3 targets for opioids
|
1) periaqueductal grey
2) retucular nucleus 3) presynaptic c-fiber receptors within the dorsal horn |
|
|
|
Describe the mechanism of opioids
|
1) bind to a g-coupled receptor (kappa, mu, delta)
2) Opening of K+ channels causes hyperpolarisation 3) Increases threshold of action potentials 4) Inhibition of Ca++ channels |
|
|
|
Describe the principal effects of the 3 main opioid receptors
|
1) mu; analgesia, euphoria, dependence
2) delta; GI motility inhibition, respiratory depression 3) kappa; sedation, miosis |
|
|
|
List the 7 features of malignant transformation
|
1) self sufficiency of growth signals
2) insensitivity to growth inhibitory signals 4) sustained replication 5) evasion of apoptosis 5) invasiveness & metastasis 6) angiogenesis 7) ineffective DNA repair |
|
|
|
List 3 classes of oncogenes and give examples of each
|
1) Excessive production of growth factors (eg PDGF (glioblastomas), TGF(sarcomas))
2) receptors eg SMO mutations causing BCC 3) signal transduction proteins eg BRAF, RAS (melanoma) |
|
|
|
Define anaemia
|
Reduction in total circulating red cell mass below normal limits
|
|
|
|
HELLP
|
Haemolysis
Elevated Liver enzymes Low Platelets |
|
|
|
List the cause and examples of normocytic normochromic anaemias
|
Deficient erythropoiesis due to hypoproliferation
1) no EPO (renal failure) 2) Aplastic anaemia (idio, drugs (anticonvulsents, chemo, antibiotics), benzene, radiation, Falconi's) 3) Pure RBC aplasia: parvovirus, anticonvulsents, organophosphates, CLL) |
|
|
|
Describe the metabolic pathway in which iron stores are increased during anaemia
|
1) Gut SI: Fe+++->Fe++ (ferric reductase)
2) Enterocytes: DMT1 -> ferritin -> transportin (inhibited by hepcidin)-> serum ferritin 3) Hepatocytes, bone marrow, spleen: store & release ferritin (serum increases during infection, inflammation to mop up free Fe) |
|
|
|
Signs and symptoms of aplastic anaemia
|
Pancytopoenia:
1) normocytic, normochromic anaemia: waxy skin, mucus membrane, pale conjunctiva, brown pigmentation, fatigue, tachy) 2) thrombocytopoenia: haemorrages (also fundi), petechiae 3) agranulocytosis: infection, sepsis, fever |
|
|
|
Causes of vertigo
|
1) Peripheral:
vestibulococclear: Meniere's, vestibular neuronitis, acoustic neuroma, drugs Labyrinth: labyrinthitis, supprutive otitis media, benign paroxsmal positional vertigo 2) Central disorders Brainstem: vertebrobasilar insufficiency, infarct Cerebellum: degeneration, tumors Other: MS, migraine |
|
|
|
Differentiate between vertigo and giddiness
|
Vertigo: spinning, nausea, difficulty walking, fear of falling
Giddiness: swimming, no nausea, can walk |
|
|
|
List the serious (red flag) disorders associated with vertigo/dizziness
|
cardiovascular: MI, aortic stenosis, arrhythmia, infarct (brainstem, vertebrobasilar)
neoplasia: posterior fossa tumour, acoustic neuroma, cancer Neurological: MS, alcohol & drugs, Parkinsons, Meniere's |
|
|
|
List the probable causes of vertigo/dizziness
|
vasovagal, postural hypotension, acute labyrinthitis, trauma, anxiety (hyperventilation), cervical dysfunction (spondylitis)
|
|
|
|
List the symptoms of hypercalaemia
|
Groans (constipation)
Stones (kidney) Bones (pain, vertebral crush fractures) Moans (psych disturbances, depression, fatigue) Davy Jones (death due to cardiac arrest, coma at levels > 3.75 mmol/L) |
|
|
|
List the organ systems affected by multiple myeloma
|
1) skeletal (2nd osteoporosis, hypercalcaemia, bone pain, fractures)
2) renal: polydipsia and dehydration due to hypercalcaemia, renal failure due to immunoglobulins congesting distal tubules (light chains in urine - Bence-Jones) 3) haematological: normo-normo anaemia made even worse by renal failure, immunodeficiency (decreased plasma cells and normal antibodies) leading to infections (pyelonephritis, resp made worse by chemo and steroids) 4) neurological: amyloids causing peripheral neuropathies, hypercalaemia effects (depression, agitation, delerium, fatigue) |
|
|
|
What are the clinical features of a melanoma lesion
|
Asymmetry
Border irregularity Colour Diameter |
|
|
|
What are the four types of melanoma
|
superficial spreading
acral lentiginous nodular lentigo maligna |
|
|
|
What is the difference between the granuloma of TC vs sarcoidosis
|
TB: central necrosis and surrounded by giant cells with horseshoe nuclei
Sarcoidosis: no necrotising centre |
|
|
|
What are the 3 diagnostic features of multiple myoloma
|
1) Bence-Jones proteins in urine, serum M-band electrophoresis
2) Bone marrow aspirate (>10% monoclonal plasma cells) 3) X-rays showing vertebral crush fractures & pepperpot lesions |
|
|
|
What is the mnemonic for the clinical features of multiple myeloma
|
CRAB
Calcium > 2.75 Renal Cr > 175 Anaemia (Hb < 10) Bone Pathology (lytic lesions, osteoporosis) |
|
|
|
Arguments for euthanasia
|
Certainty, relieved anxiety, a good death better than a painful one, frees up medical resources
Pal care can't deal with all suffering |
|
|
|
which are euthanasia
1) withdrawal of ineffective treatment 2) giving increasing analgesia simply by upping the dose of morphine 3) terminal induced coma and not providing fluids and nutrition |
All legal but documented need for increased sedation/analgesia must have clinical need to reduce suffering.
Futility is grey area, hastening death by advance health instruction OK if futile, otherwise "reasonablness" of futility based on peer agreement (Boland principle) |
|
|
|
What are the arguments against euthanasia
|
Morally wrong
Undermines trust in medical profession Slippery slope towards enforced euthanasia, benefits to 3rd parties including the state |
|
|
|
Why doesn't a doctor providing a script for a large box of endone get convicted
|
Intention, predictability, csusation very difficult to prove
|
|
|
|
Reid Sternberg cells
|
large
binucleated eosinophilic |
|
|
|
why differentiate between B & T lymphoma
|
t cell lymphpmas affect thymus
b cell lymphocytes |
|
|
|
how is a histological slide of a lymph node differentiated from a thymus
|
Germinal centres
capsules |
|
|
|
what are the histochemical markers for B and T cells
|
b is cd 20
t is cd 3 |
|
|
|
Organs commonly affected by lymphomas
|
liver, spleen, any lymphatic tissue, skin, lungs, neck, GI
|
|
|
|
symptoms of lymphoma with poor prognosis
|
fever, night sweats, weight loss
|
|
|
|
A patient presents with increasing dyspnoea and you suspect lymphoma. Describe the steps to diagnose
|
imaging, fine needle aspirate of nodes, histocytology
|
|
|
|
What happens in a splenic white pulp infarct
|
fibrotic wedge forms, not lymphoma because spleen looks otherwise normal
|
|
|
|
A spleen is dotted with small white patches. Differentials
|
TB, lymphoma (metastices or large diffuse B cell lymphomas form larger rounded masses)
|
|
|
|
Follicular diffuse white nodules in spleen. Differentials
|
heart failure (diffuse fibrosis)
TB lymphoma |
|
|
|
what are the staging criteria for lymphoma
|
Localisation v spread to other node regions
Above or below diaphragm Involvement of organs - diffuse spread |
|
|
|
What is the targeted therapy against B cell lymphomas
|
CD 20 binder RITUXUMAB
|
|
|
|
A large irregular oval white lesion with a black centre is found in the stomach mucosa. Differentials
|
H. Pylori induced lymphoma, stomach cancer, probably not ulcer if border is irregular
|
|
|
|
A mix of enlarged and normal lymph nodes are found in the mesentary. DDx
|
lymphoma
cancer infection |
|
|
|
What feaures are looked at to ask about back pain
|
site
character severity radiation allieviating/exacerbating radiation SC compression: faecal incontinance, bladder. dysfunction, leg weakness, parasthesia |
|
|
|
B symptoms
|
fever, night pain, night sweats
|
|
|
|
Neuro exam signs of spinal chord compression
|
UMN signs - hyperreflexia, Babinski, weakness, sensory lpss
|
|
|
|
Diff diag of back pain
|
spinal stenosis
disc prolapse muscle strain rheumatoid (AS, RA) |
|
|
|
Causes of ESR and rouleax
|
inflammation: fibrin, gammopathy causes reduced repulsion between RBCs
|
|
|
|
What does electrophoresis find with serum from a multiple myeloma patient
|
monoclonal antibodies showing a prominant band
|
|
|
|
what are the 3 classifications of plasma cell gammopathies
|
1) Monoclonal gammopathy of Undetermined Significance
asymptopatic myeloma with plasma cells > 10% 2) Macroglobulinaemia - hyperviscosity syndrome 3) Multiple Myeloma symptomatic with CRAB symptoms |
|
|
|
How is ALP indicative of MM
|
Elevated alkaline phosphatase associated with fractures
|
|
|
|
Why is a tecniciam scan unlikely to pick up multiple myeloma
|
binds to osteoblasts
|
|
|
|
causes of renal failure in mm
|
dehydration
light cjain toxicity forming tube casts hypercalcaemia amiloid deposits |
|
|
|
What happens with hyperviscosity syndrome with MM
|
Bleeding
blurred vision dizziness CHF Fundoscopy: antibodies cause tortuosity, blurring of disc margin. Plasmapheresis recommended as it is an emergency |
|
|
|
what are the prognostic factors of myeloma
|
B2 microglobulin (want low), albumin(want high), level of overall function/activity
|
|
|
|
MM is incurable, what are treatment goals
|
analgesia, remission, prevent bone loss with bisphosponates
|
|
|
|
treatment of renal disease with MM
|
rehydration
rapid initiation of chemo and prednisone dialysis |
|
|
|
What is the typical treatment regimen of MM
|
chemo
high dose single agent chemo radiation of single lesions autolagous stem cell transplants new therapies: Bortezomib (proteosome inhibitor - prevents breakdown if ikappaB), thalidomide (improves survival in elderly), Revlimid (lenalomide) |
|
|
|
What are the 3 mechanisms of pernicious anaemia
|
Binding to IF, parietal cells, block the complex binding to ilial receptors
|
|
|
|
3 types of autoimmune disease
|
vitiligo
Hashimoto's thyroiditis pernicious anaemia |
|
|
|
Geriatric giants
|
instability
iatrogenic incontenence intelligence |
|
|
|
What are the steps of medicare overservicing
|
Medicare investigates
professional standards authority investigates (has lay person, medical and other health practicioner) determining authority determines penalty (reprimand, counselling, repayment) appeals can go to court |
|
|
|
What are the 3 mechanisms of pernicious anaemia
|
Binding to IF, parietal cells, block the complex binding to ilial receptors
|
|
|
|
3 types of autoimmune disease
|
vitiligo
Hashimoto's thyroiditis pernicious anaemia |
|
|
|
Geriatric giants
|
instability
iatrogenic incontenence intelligence |
|
|
|
What are the steps of medicare overservicing
|
Medicare investigates
professional standards authority investigates (has lay person, medical and other health practicioner) determining authority determines penalty (reprimand, counselling, repayment) appeals can go to court |
|
|
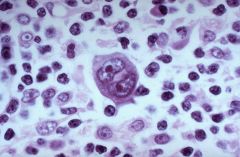
What is this, what are they indicative of
|
Reid Sternberg cells
Hodgkin's Lymphoma |
|
|
|
A stage II Hodgkin's Lymphoma would occur where? What about stage III
|
In more than one region above the diaphragm eg bilateral cervical + axilla.
Stage III would involve nodes below the diaphragm +/- spleen, liver |
|
|
|
Why does ALL initially involve the bone marrow
|
ALL is a precursor B/T lineage malignancy producing abnormal blast cells, therefore, it originates in bone marrow with ALL-BB cells and the thymus with ALL-T
|
|
|
|
Which enzymic marker is present in ALLto differentiate it from AML
|
Terminal deoxytransferase is produced by B and T cell blasts
|
|
|
|
Explain how pre-T cells can produce ALL with no thymic involvement
|
NOTCH1 signalling mutations allow T-cell blasts to proliferate outside of the thymus, ie in the bone marrow
|
|
|
|
What classification of lymphoid neoplasm is CLL
|
Peripheral B cell, originating in lymph nodes and later disseminating to bone marrow, spleen and liver
|
|
|
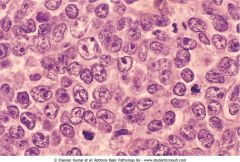
What is this, describe it's aetiology and typical course of the disease
|
Diffuse B cell lymphoma. Agressive, disseminates to other nodes and later, extranodally. Initially localised to lymph nodes and treatable.
|
|
|
|
Describe the relative iron storage and iron storage capacity of the different types of microcytic anaemia
|
Iron deficiency: low ferritin, high SC
Chronic disease: high ferritin, low SC Sideroblastic: high serum Fe Thallesaemia: |
|
|
|
What is expected of ferritin levels when B-thallasaemia is first diagnosed and after treatment with packed cells
|
Starts normal then increases because serum ferritin mirrors total body iron stores
|
|
|
|
Describe the TB granuloma
|
horseshoe nucleated macrophages surrounding necrotic centre
|
|
|
|
how can you differentiate between hydatid v abcess in liver
|
Abcess has fibrotic cover, hydatid cysts form distinctive gelatinous coat
|
|
|
|
What are the diffs of brain petichiae
|
malaria, fat embolism causing multiple infarcts
|
|
|
|
A blood film shows signet ring infestations
|
falciparum merizoids
|
|
|
|
what is the clinical outcome of malaria (think renal)
|
Merizoids release enzymes causing haemolysis releasing haemoglobin leading to depletion of ? And renal failure
|
|
|
|
A 25 yo male person has loose, non bloody loose motions for 7 days after recent travel to Thailand. Differentials
|
Gastroenteritis (virus, bacteria, amoeba)
IBD HIV |
|
|
|
how to diff between diarrhoea between giardia, cholera, entamoeba, coeliac
|
consistency of stool, giardia cysts, biopsy for coeliac
|
|
|
|
what do villi look like with giardia
|
deep crypts producing elongated SI villi
|
|
|
|
Explain the lifecycle of amoeba
|
Faecal oral, human hosts only, forms cysts, migrates to liver forming abcesses requiring aspiration
|
|
|
|
What are the symptoms and life cycle of schistosomiasis
|
Cercaria penetrates skin (swimmers itch) can enter cns, enters bladder and urine reproduces forming merocydium, can enter transitional bladder epithelium causing chronic inflammation, squamous cell metaplasia, bloody urine
|
|
|
|
12 yo girl on pig farm, seizures, neck stiffness, drowsiness and brain mri shows calcification nodules areas. Diagnose
|
calcification from eosinophilia due to helminth. Tenia Soleum tapeworms form cysts
|
|
|
|
with cystacercosis and tenia soleum, which are the intermediate and definitive hosts
|
pig is intermediate host (non reproductive phase) and human is definitive (sexually mature and reproducing phase)
|
|
|
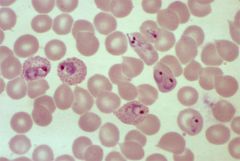
What is this
|
P.vivax infected erythrocytes
|
|
|
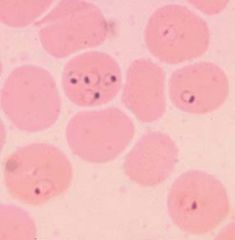
Describe this
|
Erythrocytes infected with P.falciparum showing signet rings
|
|
|
|
What are the signs of typhoid
|
Non specific febrile illness with no pathognomonic sign but severe abdo pain, fever, bloody diarrhoea. Treat empirically with quinolones
|
Typhoid fever is characterized by a slowly progressive fever as high as 40 °, profuse sweating and gastroenteritis. Less commonly, a rash of flat, rose-colored spots may appear.
Classically, the course of untreated typhoid fever is divided into four individual stages, each lasting approximately one week. In the first week, there is a slowly rising temperature with relative bradycardia, malaise, headache and cough. A bloody nose (epistaxis) is seen in a quarter of cases and abdominal pain is also possible. There is leukopenia, a decrease in the number of circulating white blood cells, with eosinopenia and relative lymphocytosis, a positive reaction and blood cultures are positive for Salmonella typhi or paratyphi. The classic Widal test is negative in the first week.. In the second week of the infection, the patient lies prostrate with high fever in plateau around 40 °C and bradycardia (sphygmothermic dissociation), classically with a dicrotic pulse wave. Delirium is frequent, frequently calm, but sometimes agitated. This delirium gives to typhoid the nickname of "nervous fever". Rose spots appear on the lower chest and abdomen in around a third of patients. There are rhonchi in lung bases. The abdomen is distended and painful in the right lower quadrant where borborygmi can be heard. Diarrhea can occur in this stage: six to eight stools in a day, green with a characteristic smell, comparable to pea soup. However, constipation is also frequent. The spleen and liver are enlarged (hepatosplenomegaly) and tender, and there is elevation of liver transaminases. The Widal reaction is strongly positive with antiO and antiH antibodies. Blood cultures are sometimes still positive at this stage. (The major symptom of this fever is that the fever usually rises in the afternoon up to the first and second week.) In the third week of typhoid fever, a number of complications can occur: Intestinal hemorrhage due to bleeding in congested Peyer's patches; this can be very serious but is usually not fatal. Intestinal perforation in the distal ileum: this is a very serious complication and is frequently fatal. It may occur without alarming symptoms until septicaemia or diffuse peritonitis sets in. Encephalitis Neuropsychiatric symptoms (described as "muttering delirium" or "coma vigil"), with picking at bedclothes or imaginary objects. Metastatic abscesses, cholecystitis, endocarditis and osteitis The fever is still very high and oscillates very little over 24 hours. Dehydration ensues and the patient is delirious (typhoid state). By the end of third week the fever has started reducing this (defervescence). This carries on into the fourth and final week. |
|
|
describe the life cycle of schistosomiasis
|
Liver flukes, 2nd most prevalent parasite to malaria, water borne via molluscs as intermediate host, acquired by swimming, ceccarial penetration and dermatitis, human definitive host, enters bladder (dysplasia) or GI then liver (cirrhosis)
|
|
|
|
what is the treatment for schisto
|
priziquantal
|
|
|
|
Chagas disease
|
3rd most important parasitic disease (poor housing), causitive T.cruzi transmitted by faeces following beetle bite (causes iron loss anaemia), flu like acute plase and inflammation, 20 years incubation then arrhythmias ( paeds treated with abs but adult only effective treatment is pacemakers)
Causes achlasia and megaoesophagus |
|
|
|
African trypanosomiasis
|
Sleeping sickness; tsetse flies, lymphadenopathy with trypanosomes on aspiration, drowsiness and delerium. Treatable with specific ab
|
|
|
|
Leishmaniasis
|
sand flies causing cutaneous ulcers and later, visceral disease. Treated with iv amphotericin or antimony locally
|
|
|
|
ascaris lumbricoides
|
pin worms, can block gi with overwhelming colonisation, does not penetrate mucosa
|
|
|
|
Leprosy
|
tuberculoid causes red numb papular lesions still presrnt in northern australia
|
|
|
|
plague
|
gram -ve bacteria, transmitted by fleas on rats, produces buba that, if they burst internally, causes massive septic shock and death
|
|
|
|
what are the endemic tropical diseases in austrlalia
|
buruli, meliodosis, scrub typhus
|
|
|
|
Dengue
|
spread by aedes aegypti mos, haemorragic fever develops after 2 strains (4 in all) within days, muscle weakness, rheum pain, fever for a week, petichiae in haemorragic fever (tornique test)
|
|
|
|
The 6 I's of travel medicine
|
immunisations
immersion insurance indiscretions ingestion |
|
|
|
If a young person presents with fever, what are the considerations
|
travel v non travel cause: know incubation times, travel hx, compliance with prophylactic meds
|
|
|
|
What are the red flags for diarrhoea
|
faecal incontinance, abdo pain, blood, mucus, fever, cancer constitutional symptoms
|
|
|
|
What is the incubation stage of malaria
|
2 weeks within the liver, asymptomatic
|
|
|
|
describe the malaria cycle
|
Anopheles bite injects sporozoites
2 weeks in liver then release of merozites blood cycle sormation of sporozytes and transfer back to mosquitoes |
|
|
|
Why is Australia free of falciparum malaria
|
Takes time for Falciparum to sexually reproduce in humans forming sporozytes
Requires "triangle" of transmissability ie human, mosquito, ? |
|
|
|
malaria diffs
|
influenza, typhoid, typhus, heat exhaustion
|
|
|
|
What are the possible courses of malaria
|
cerebral malaria arises from vascular adhesion causing infarcts (delerium, coma, seizures) note febrile seizure is diff
Severe anaemia: Hb < 5 causing high output heart failure, MI Overwhelming infection; death from sepsis and septic shock placental malaria: placenta is attacked preferentially causing intrauterine growth retardarion |
|
|
|
a patient from Townsville presents with fever and polyarthritis, treat
|
self limiting Ross River, treat symptoms
|
|
|
|
what are the symptoms of yellow fever
|
haemorragic fever, black vomit, fever - African, not Asian disease
|
|
|
|
A person presents with febrile rash, headache, depression and had travelled to the tropics. Possibles?
|
dengue, measles, meningitis
|
|
|
|
scrub typhus
|
Spread by small arthropods, common in Australia, a type of rickettsia, treated by doxy
|
|
|
|
What cells are derived from pluripotent stem cells
|
Pro-erythrocytes -> reticulocytes -> RBC
Lymphoid stem cells -> lymphoblast -> B & T cells Myeloid stem cell -> monoblast (monocytes) or megacaryoblast (platelets) or myeloblast -> band cells -> granulocytes (neutrophils, basophils, eosinophils) |
|
|
|
How do basophils appear stained, what is their role, what do they produce
|
1) blue granules
2) allergies, asthma 3) histamine, heparin, leukotrienes |
|
|
|
How do eosinophils appear stained, what is their role, what do they produce
|
1) pink
2) response to helminths, protozoa 3) histamine and basic protein |
|
|
|
A leukocyte with a lobed nucleus and azurophilic granules. What is it. What is it for.
|
1) Neutrophils
2) Acute inflammation |
|
|
|
What is the role of CD4
|
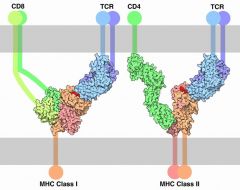
It is a co-receptor for MHCII and lives on helper T cells and antigen presenting cells. It binds at the same time to MHCII together with the T cell receptor, amplifying the signal in the Th cell.
|
|
|
|
List the categories of skin pigmentation disorder
|
1) hyperpigmentation
2) epidermal melanocyte proliferation 3) benign neoplasia (naevus formation) 4) dermal melanocyte infiltration 5) dysplastic naevae 6) melanoma |
|
|
|
What are the 3 main types of non dysplastic naevae
|
junctional
dermal compound |
|
|
|
What are the clinical features of a compound naevus
|
dark, 3-8 cm elevated round (but may be irregular)
histologically shows normal melanocytes pushing up the epidermal junction and also present at the epidermal junction |
|
|
|
What are the risk factors for developing melanoma
|
9p21
Fitzpatrick types 1,2 Blistering sunburn < 20yo numerous freckles, dysplastic naevae immunosupression |
|
|
|
List the 4 phases of melanoma development
|
1) in situ dysplasia (lentiginous nevus or dysplastic nevus) and proliferation at the basal layer
2) radial growth 3) penetratiion of the basement membrane into the dermis and vertical growth phase |
|
|
|
List 4 aetioloigies of microcytic anaemia
|
1) iron deficiency (blood loss, diet, absorption, pregnancy)
2) thalassaemia 3) chronic disease (autoimmune, infection, cancers) 4) sideroblastic |
|
|
|
List 4 aetiologies of normocytic anaemia
|
1) renal failure
2) chronic disease 3) haemolysis 4) aplastic anaemia |
|
|
|
List the steps of the mechanim of the effect B12 deficiency on erythropoieisis
|
1) dietary folate remains in unusable form (not converted to FH4)
2) Impaired DNA synthesis and replication of erythroblasts is arrrested 3) RNA and protein synthesis continues, swelling reticulocytes 4) fewer (ie anaemia) and macrocytic reticulocytes are formed |
|
|
|
What are the histological features of macrocytic anaemia
|
1) blood exam shows pancytopoenia
2) large neutrophils with hypersegmented nuclei 3) RBC show anisocytosis (wide variation in size & shape) 4) low reticulocyte count |
|
|
|
What iron studies differentiates between iron deficiency anaemia and caused by chronic disease
|
1) serum ferritin: lower in iron deficiency and raised in chronic disease (bone marrow has lots of iron but cannor be accessed)
2) serum iron (low vs normal) 3) TIBC (high with deficiency and normal with chrronic disease) |
|
|
|
Erythropoiesis is stimulated principally by which of the following?
A. Reduced pulmonary venous oxygen tension B. Reduced oxygen tension in renal arterial blood C. Reduced firing of a chemoreceptor in the carotid arteries D. Reduced secretion of an inhibitory adrenocortical hormone. |
B) reduced oxygen tension in renal arterial blood triggers paratubular cells to secrete EPO
|
|
|
|
Which of the following could be a causal factor(s) for a decreased haemoglobin concentration in a patient with renal disease?
A. decreased erythropoeitin production B. haemolysis C. iron deficiency D. haemodilution E. all of the above |
Renal disease:
A) decreased EPO B) haemolysis can cause renal failure & C) iron deficiency D) haemodilution possible witth renal disease E) correct answer |
|
|
|
Why is iron deficiency anemia a problem in developing countries
|
Diet deficient in meat ie haem iron is absorbed more efficiently than non-haem iron obtained from vegetables
|
|
|
|
What are hormonal causes of hypercalcaemia
|
Hyperparathyroidism
Hyperthyroidism Adddison's disease |
|
|
|
How does PTH increase serum Ca
|
PTH->kidneys->calcitriol->increased intersinal absorption
Increased reabsorption of calcium in kidneys Increased osteoclast/decreased osteoblast activity |
|
|
|
What are the clinical signs of hypocalcaemia
|
Vomiting, nausea, diarrhoea, weakness, coma, nervousness, tetany, laryngeal spasm, cramps
|
|
|
|
What are the diagnostic features of MM
|
Two out of 3 of:
1) Elevated M in serum and/or BJ proteins in urine 2) > 10% plasma cells in bone marrow 3) radiological evidence |
|
|
|
What are the prognostic features of MM
|
1) renal failure due to tubular clogging
2) fractures and hypercalcaemia 3) anaemia due to crowding out of bone marrow by plasma cells |
|
|
|
What is the mechanism by which MM causes bone loss
|
1) MM plasma cells bind to stroma
2) secrete OAF and trap OPG which inhibits TRANCE 3) OAF activates osteoblasts to secrete TRANCE 4) Increased TRANCCE and decreased OPG stimulated osteoclasts and inhibits osteoblasts |
|
|
|
What blood tests should be ordered to investigate MM
|
1) FBC (normocytic, normochromic anaemia
2) ESR: hypergammaglobinaemia causes roulade formation 3) 24 hr urine for BJ proteins, serum electrophoresis for IgG, M protein (monoclonal gammopathy) 4) U&E (hypercalcaemia, raised creatinine and urea, nitrogen, urate) 5) Bone marrow aspirate (> 10% abnormal plasma cells |
|
|
|
What are the important history questions regarding the natureof back pain
|
Nature (throbbing=inflammation, boring=bone, superficial=muscular, deep=referred (dysmenorrhoea, kidneys)
|
|

