![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
123 Cards in this Set
- Front
- Back
|
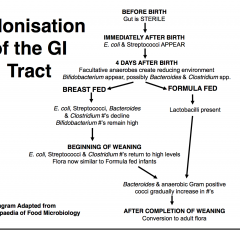
Colonization of the GI tract
|
|
|
|
Normal Commensal Flora of the GI Tract
- Mouth: 10^10 bacteria (>500 species) |
- Streptococcus, Neisseria, Actinomyces, Veillonella &
Lactobacillus, some yeasts, transient viruses - Eruption of 1st teeth: Porphyromonas, Prevotella & Fusobacterium - Growth of Teeth: S. sanguis*, S. mutans* & S. salivarius *Dental plaque |
|
|
Normal Commensal Flora of the GI Tract
Stomach: Sterile (0 bacteria/ml) or 103 bacteria/ml |
– Streptococcus, Staphylococcus, Lactobacillus &
Peptostreptococcus - Helicobacter pylori: Gastritis; Peptic/duodenal ulcers N.B. 105-107 bacteria/ml: - abnormality (achlorhydria or malabsorption syndrome) |
|
|
Normal Commensal Flora of the GI Tract
- Small Intestine: Sparse - Duodenum: 0-104.5 bacteria/ml |
- (increases distance away from stomach)
Duodenum: Fluctuating transients: aerobic Streptococci, Staphylococci, Lactobacilli, yeasts N.B Complete absence of coliforms & Bacteroides |
|
|
Normal Commensal Flora of the GI Tract
- Jejunum-Ileum: 0-105/ml - 107 bacteria/ml - AT Ileocecal junction 106 - 108 organisms/ml - Large Intestine: Colon 1010 - 1012 bacteria/ml (richest & complex >400 species identified) |
– High counts Enterobacteriaceae – some Streptococcus, Staphylococcus, Lactobacillus, Bacteroides, Bifidobacterium, Clostridium
Large gut: 95-99% Anaerobic: Bacteroides, Bifidobacterium, Eubacterium, Peptostreptococcus & Clostridium Plus Enterobacteriacea |
|
|
Factors Affecting Microbial Composition
ALLOGENIC: originate outside ecosystem - E.g., “Western” diet vs Native, vegetarian diet (Africa) Inc Bacteroides, Dec enterococci (other aerobes) |
- Diet: nature of meal (gastric emptying); milk
AGE, GEOGRAPHIC REGION ANTIBIOTIC THERAPY: removal of normal flora (colonization by pathogenic org’s E.g., C. difficile) Surgery: Alters bacterial pop. - Ileostomy effluents - unique ecological niche NOT corresponding ileum or colon |
|
|
Factors Affecting Microbial Composition
AUTOGENIC: arise within ecosystem - Environment: opt growth 37oC, anaerobic |
H+ concentration (gastric acid) Peristalsis (directory flow rate), Shedding of epithelium, Mucus, Conjugated Bile salts? Immunological response (IgA)?
|
|
|
ii. Activities of Microorganisms:
|
Nutritional competition, Prodn bacterial inhibitors: bacteriocins, antibiotics Toxic metabolic end products
H2S prodn, Competition for attachment sites Maintenance of low oxidation-reduction potentials |
|
|
I. Upper GI Infections: Oral Disease (Herpes & Mumps
II. Lower GI Tract Infections - (Food/Water) |
I. – Dental Caries – Periodontal disease – Abscess – Systemic Infection
II. – Changes in human demographics – Changes in food preferences – Changes in food production – Changes in food distribution – Microbial adaptation |
|
|
Food Hazards
- U.S figures ONLY include Botulism, E. coli O157:H7(STEC), Cholera, Salmonellosis, Typhoid fever, Shigellosis, Listeriosis (from 2002) & Vibriosis (from 2007) |
1. Microbial contamination
2. Naturally occurring toxicants 3. Environmental contaminants (e.g., metals) 4. Nutritional problems (i.e., malnutrition, undernutrition) 5. Pesticide residues 6. Food additives |
|
|
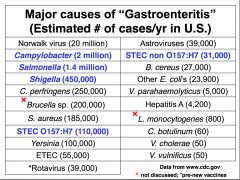
Major causes of "gastroenteritis"
- Norovirus (49%) - Bacteria (40%) |
|
|
|
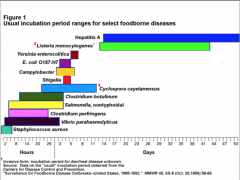
Route of infection: Entery, disease, Exit
- Incubation period |
|
|
|
FOOD-POISONING: TOXEMIA
FOOD-ASSOCIATED INFECTIONS (food-borne) |
consumption of food containing toxins (chemical or microbial) C. botulinum, S. aureus, B. cereus (1 form)
Fungal & Marine toxins consumption of food containing organism (acts as vehicle for entry) A wide variety of pathogens |
|
|
Enteritis
“Gastro” enteritis Colitis Enterocolitis Dysentery Diarrhoea |
inflammation of intestinal mucosa
inflammation of stomach & intestinal linings inflammation of large intestine inflammation of small & large intestine inflammation of GI tract with blood & pus in faeces frequent and/or fluid stool (>3 loose stools) - Acute, Persistent, Chronic |
|
|
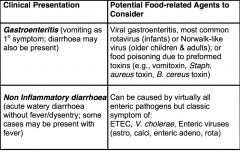
Etiologic Agents to Consider for Various Manifestations of Food Related Illnesses
|
|
|
|
Types of Diarrhoea
|
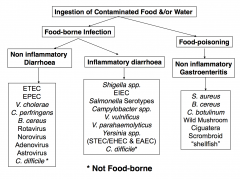
|
|
|
Food Toxemia: “Food Poisoning” “Non-inflammatory Gastroenteritis”
|
Aetiological Agents
• Bacterial: S. aureus, B. cereus (1 type) & C. botulinum • Fungal: Wild Mushrooms (Amanita, Clitocybe & Psilocybes) Aflatoxin (Aspergillus sp.) • Marine/Algae: Ciguatera, Scromboid & “Shellfish” |
|
|
Food poisoning:Toxemia Ingestion of preformed toxins (NOT INFECTION) NO microbial growth within human GI tract
|
Symptomology: usually rapid (minutes-hours)
(C. botulinum: 6hrs-8days) Lack of fever; no faecal leukocytes Toxins Affect: CNS (C. botulinum) Both CNS & Intestines (S. aureus & B. cereus) |
|
|
Staphylococcus aureus: Gram +ve cocci (0.5-1.5mm)
Arranged in singles, pairs & clusters Aerobic or facultative Coagulase +ve, Catalase +ve, ST Enterotoxin production Mode of action: UNKNOWN - act on gut receptors; stimulate vomiting (vagus & sympathetic nerves) – NO stimulation of adenylate cyclase |
Habitat: Human & animal pathogens (skin), 50% humans carry Staphylococci
8 Exotoxins (A, B, C1, C2, C3, D, E, H) Water-soluble, low mw proteins, ST (chromosomal) Infective dose 105 – 108orgs/g food (A & D common) Neurologic (vomiting) & Enteric (diarrhoea) effect |
|
|
Staph Aureus: Clinical symptoms
|
- Self-limiting illness: EMESIS within 6hr ingestion (mean 4.4hr) (BUT Not all vomit) - Recovery 24-48 hours
nausea, abdominal cramps, diarrhoea (watery), headaches, muscular cramping and/or prostration |
|
|
Staph Aureus - Incriminated Foods:
Poor Handling of Food Outbreaks Incidence |
cooked meat (fish, poultry), bakery foods (cream-filled),
dairy produce, fruit, vegetables & salads. – Self-limiting disease (no incentive to report) – US National Surveillance System (1-5% cases) Highest: Summer, 2nd: November/December: Holiday |
|
|
Staph Aureus: Isolation & Identification
|
Variety of Media available (MSA)
Baird-Parker(selective, diagnostic, recovery) - Lithium chloride & tellurite (selective agents) Egg yolk & pyruvate (recovery) Reduction of tellurite shiny, jet-black colonies Surrounded by clearing zone Confirm: Coagulase test |
|
|
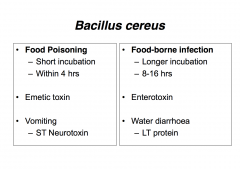
Bacillus cereus: Habitat: air, soil, water & dust
Gram +ve rods (0.7mm x 3-10mm long) Arranged in chains, Aerobic or facultative, Spore former, Emetic toxin & Enterotoxin Easily spread to food: Cross-contamination • 2 types of Gastroenteritis |
Emetic (vomiting): Resembles Staph. aureus Short incubation: 2-3 hrs, Duration: 6-24 hr, ST Neurotoxin (peptide) prodn by cells in food
Incriminated Foods: rice & pulses, Incidence is under-reported: mild illness 27,000 cases/year (may include diarrhoeal type) |
|
|
Bacillus cereus: Isolation & Identification
|
Implicated food contains >105 org/g
• Non-selective medium: Blood agar (sometimes + polymyxin (suppress Gram-ve) |
|
|
Clostridium botulinum: Variable size Gram +ve rods Anaerobic, Ferment range of CH2O’s Gas Spore former, Produce exotoxins Susceptible to penicillin
Habitat: soil (fertilized animal excreta), lower GI tract humans & animals |
Botulism - Food poisoning (1800’s)
• Infant (1976): Most common botulism in U.S • Food poisoning Association - Originally: contaminated meat (sausage) Now: Home-canning, vegetables, fish, fruits & condiments • Major concern food processors & consumers |
|
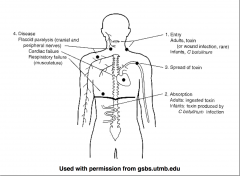
Clostridium botulinum: pathogenesis
|
NEUROTOXIN
• 8 types (A, B, C1, C2, D, E, F, G) Proteins - Toxin A (potent) 10-8g KILL HUMAN • Humans: A, B, E, rarely F (Animals: C & D) • U.S frequent isolate type A, then B & E • Europe frequent isolate type B (A rare) |
|
|
Food poisoning Botulism: Clinical Symptoms Vary
- Mild illness (disregarded or misdiagnosed) - Serious disease (fatal within 24hr) GI disturbances 1/3 patients (toxin A or B) & Almost all toxin E |
Incubation time: 18-36hr, Dependant: amount & antigenic toxin type Includes: nausea, vomiting & abdominal pain Diarrhoea often present: Constipation may occur
Toxemia symptoms then apparent, No fever in absence of complicating infections |
|
|
Clostridium botulinum: Diagnosis
• Fatal toxemia (rule out botulism) • REPORTABLE DISEASE |
Presumptive diagnosis – Presence of rapidly descending paralysis – History of ingestion of home canned or fermented food?
Confirmative diagnosis – Demonstration of botulinum toxin in serum/faeces or incriminating food (mouse toxin-neutralization test) |
|
|
Differential Diagnosis
- Guillain-Barré syndrome: ascending paralysis - Myasthenia gravis: descending paralysis - Other microbial food poisonings & gastroenteritis - Chemical (& non-microbial) food poisonings |
GB: Paresthesias or other sensory abnormalities, elevated cerebral spinal fluid protein
MG: Accentuation of muscle fatigability during exercise and positive response to endrophomium No cranial nerve involvement, Symptoms occur within minutes |
|
|
Infant Botulism - NOT Food Poisoning
- Infants (2 weeks-6months age) - Implicated Types A & B - Symptoms: illness & constipation (overlooked) - Later: Head control lost, Infant becomes flaccid - Severely Infected: Respiratory Arrest |
Spore Germination (GI tract) - Vegetative cells - replicate & release toxin
Proceeds: lethargy, sleeps more than normal Suck & gag reflexes diminish, Dysphagia becomes evident as drooling 4-15% cases sudden death |
|
|
Clostridium botulinum: Diagnosis & Treatment
Diagnosis: is difficult Requires: prompt action for survival - Differential Diagnosis: neurological + GI Toxin - Demonstration in faeces |
Treatment
Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G)-(Equine) Supportive measures: maintain respiration Baby Botulism Immune Globulin (BIG-IV) for A & B toxins |
|
|
Mushroom (Fungal) Toxin: Not common
- Short-acting: Wild mushrooms - Long-acting: Mushrooms (uncultivated) - CAN BE FATAL |
Short: Toxin: Museinol, Muscarine, Psilocybin, Coprius artemetaris, Ibotenic acid, Incubation <2hrs: Vomiting, diarrhoea
Toxin: Amantia, Incubation 4-8hrs: Diarrhoea, abdominal cramps |
|
|
Mycotoxigenic Fungi
Contamination of: - Tree nuts, peanuts, oilseeds (corn & cotton) Responsible for: - Acute necrosis, cirrhosis & carcinoma (liver) |
Mycotoxins: 2 metabolites; Aspergillus, Fusarium & Penicillium
AFLATOXINS: Aspergillus flavus & A. parasiticus - favourable conditions (temp & humidity) |
|
|
Marine Toxins: Large predatory reef fish: barracuda, grouper & amberjacks
Neurologic symptoms: circumoral & extremity paresthesia, severe pruritus, hot/cold temp reversal |
Ciguatera poisoning: Caribbean/Tropical Pacific
Dinoflagellates: Gambierdiscus toxicus: Ciguatoxin Acute GI symptoms: 3-6hrs after ingestion - Watery diarrhoea, nausea, abdominal pain (12 hr) |
|
|
Scromboid poisoning: Non-allergic histamine
Scrombridae Fish: tuna, mahi-mahi, marlin & bluefin Other symptoms: dizziness, urticaria (rash), facial flushing, generalised pruritus, paresthesias |
Bacteria: Stenotrophomonas maltophilia, M. morganii
Histadine - Histamine (Scrombotoxin - Burning sensation in mouth, a metallic taste Acute GI symptoms: mins-3hrs after ingestion (<1hr) - - Watery diarrhoea, nausea, lasting 3-6hrs |
|
|
Neurologic & Paralytic Shellfish Poisoning
- Dinoflagellate algae: Karenia brevis |
Brevetoxins
• Incubation: <1-3 hours; Duration: 24-73 hours • Paresthesia, mouth numbness, tingling sensation of mouth & extremities, GI upset |
|
|
Paralytic shellfish poisoning
- Tingling & numbness of mouth spreading to extremities, GI symptoms less common, ataxia (muscular in-coordination) Severe cases: muscular paralysis, respiratory paralysis |
Dinoflagellate algae: Alexandrium spp., Gymnodinium catenatum, Pyrodinium bahamense, Gonyaulax spp.
• Saxitoxins: Incubation: <2hrs; Duration: 3 days |
|
|
Non-Inflammatory Diarrhoea (Aetiological Agents)
• Bacterial – E. coli (ETEC; EPEC), V. cholerae, Clostridium perfringens, Bacillus cereus (diarrhoeal) • Viral- Rotavirus, Noroviruses, Adenoviruses, Others - Toxins Affect: Enterotoxins (bacteria) |
Food associated: foodborne infection
Ingestion of organisms in food, Toxins produced (bacteria) (NO bacterial INVASION) Symptomology: Acute watery diarrhoea Longer incubation (than toxemia) due to colonisation With/without fever |
|
|
Escherichia coli: Member of normal (commensal) intestinal flora
- Family Enterobacteriacea, Gram -ve bacilli (Rods) Facultative anaerobes - Biochemical reactions, Complex antigenic structure - Prodn variety of toxins (virulence factors) - major opportunistic pathogen |
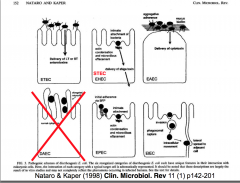
|
|
|
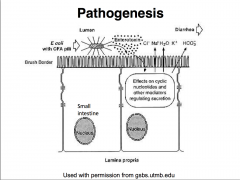
ENTEROTOXIGENIC E. coli (ETEC)
Transmission: contaminated food & water Infective dose: 100 million - 10 billion cells 1st cause of “Traveller’s Diarrhoea” 20-55,000 cases/year in U.S. |
|
|
|
2 Plasmid-encoded ENTEROTOXINS
LT: mw80,000; Similar to Cholera; adenylate cyclase ST: mw1,500-4,000; guanylate cyclase |
|
|
|
ENTEROPATHOGENIC (EPEC)
“Infantile Diarrhoea”: Childhood Diarrhoea Developing countries (50% mortality) • Management: Rehydration therapy • Antibiotic therapy: Trimethoprim/Fluoroquinolones |
Pathogenesis
- NOT fully understood (No ST or LT or CFA) - Plasmid-borne (EAF) Bundle-forming Pilus (BFP) - Effacement of microvilli |
|
|
Vibrio cholerae: Family Vibrionaceae
– Single curved Gram-ve rods, 2 - 4mm long – (May be linked end to end) forming “S” shapes – Motile (single polar flagellum) – Non-spore forming – Oxidase +ve |
– O & H antigens
• Serogroup: O1 & O139 – Ferment sucrose & mannose NOT arabinose – Acid sensitive – Halotolerant |
|
|
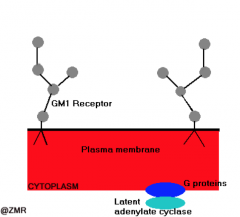
Vibrio cholerae: pathogeneis
- Infective dose: 108 - 1010 orgs - Vibrio cells aligning close to microvilli of SI - CHOLERA TOXIN - Bacteriophage encoded, AB toxin |
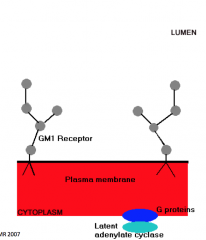
Receptor: Ganglioside GM1
|
|
|
Vibrio cholerae: pathophysiology
- Result: Diarrhoea occurs (20-30 L/day) “RICE WATER STOOL” |
|
|
|
Vibrio cholerae: treatment
|
Management: REPLACE IONIC LOSS
- Oral and/or IV admin glucose Antibiotic therapy: Tetracycline Reduce duration diarrhoea Prevention: Parentally administered vaccine (109 killed Vibrio cells) - Lasts 3-6 months Only effective O1 serotype - Not Recommended by FDA Sanitation & Hygiene |
|
|
Vibrio cholerae: Diagnosis
- Haiti (after earthquake) - Sucrose +ve V. cholerae - Sucrose -ve V. parahaemolyticus V. vulnificus - Cholera antigens: Serotypes: Inaba, Ogawa & Hikojima, Biotypes: “Classic” & El Tor |
• Clinical presentation (Cholera)
• Screening of stool samples Oxidase activity • Thiosulphate-citrate-bile salts-sucrose (TCBS) agar Sucrose (differentiating agent) |
|
|
Clostridium perfringens: 2 different diseases
1. Necrotic enteritis (Darmbrand & Pig-Bel) - RARE (Papua New Guinea) C. perfringens strain type C 2. Type A food-borne infection - Major cause food-borne infection in U.S C. perfringens strain type A |
Diagnosis
• Case history & symptoms • Large # (>106/g) C. perfringens spores in faeces • Large # vegetative cells (same serotype) in incriminated food (>106/g) • Presence of enterotoxin in faeces |
|
|
Bacillus cereus: Diarrhoeal (1948):
Resembles C. perfringens Characterization: diarrhoea & abdominal pain Incubation: 8-16hrs, Duration: 12-24 hr LT Enterotoxin(s) prodn vegetative growth (late exponential phase) (in SI) adenyl acyclase-cAMP |
(Foods: meat & vegetable dishes, sauces, pasta, desserts & dairy products)
|
|
|
Parasitic Causes of Diarrhoea
|
Cryptosporidium spp.
Cyclospora cayetanensis Entamoeba histolytica Giardia intestinalis |
|
|
Viral Causes of Diarrhoea
|
Rotavirus
Noroviruses (Norwalk & Norwalk-like Viruses) Adenovirus Small round structured Astroviruses Hepatitis A & E |
|
|
Rotavirus: Family Reoviridae: Respiratory Enteric Orphan
- 8 Genera Wheel-shape, 70nm dia, Non-enveloped - 11 segments ds RNA (6 Structural, 5 Non-structural) - 10 human rotavirus serotypes (G1-G4 important) |
![Common genotypes: G1, 2, 3, 4 & 9 with [P8] & [P4]
Group 1: Worldwide distribution
Non-group 1 (2,3): Limited distribution (2 - China)](https://images.cram.com/images/upload-flashcards/87/17/88/4871788_m.png)
Common genotypes: G1, 2, 3, 4 & 9 with [P8] & [P4]
Group 1: Worldwide distribution Non-group 1 (2,3): Limited distribution (2 - China) |
|
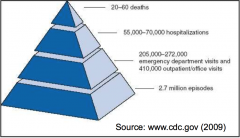
Rotavirus Surveillance/year
Asia, Africa & LatinAmerica - 140 million cases, >870,000 deaths (severe dehydration & electrolyte loss) |
• Factors for High Incidence & Mortality
– Unsafe water – Inadequate sanitation Age: <6months & >5 years ASYMPTOMATIC Protection against diarrhoeal infection |
|
|
Rotavirus: Family Reoviridae: Pathogenesis
- Seasonal? Temperate, Developed countries: “Winter Gastro” Tropical, Developing countries: Year long (Summer) |
Transmission: Faecal-oral route, Water & Air-borne
Incubation period: <48hrs (1-3days) Replication: epithelial cells of SI • Faeces: 108-1010 virus particles/ml, Shedding MAY persist for 10 days or more Peak within 8 days |
|
|
Rotavirus: Family Reoviridae: Pathogenesis
|
Histopath studies: shortening & blunting of villi patchy, irregular intact mucosa mononuclear cell infiltration of lamina propria
Diarrhoea results from the loss of absorptive area & the flux of water/fluid across damaged surface |
|
|
Rotavirus: Family Reoviridae: - Clinical Manifestations
Detection- Virus in stool (peak at day 3/4 of diarrhoea) • Latex agglutination, EIA (for characterisation),, Electron Microscopy (labour intensive, insensitive), Electrophoresis of RNA segments |
Illness: Sudden onset watery diarrhoea
- With/out vomiting - Up to 6 days (longer: immunocompromised) Complications - Dehydration, severe & life-threatening |
|
|
Rotavirus: Family Reoviridae: Vaccines
- 1st Rotavirus Vaccine (Rotashield®) |
Rotashield: Live Oral Tetravalent Vaccine, 3 x 2.5ml doses @ 2, 4 & 6 months of age
- Prevent 50% rotavirus cases 70% severe cases; 100% associated dehydration - Adverse Reactions: Intussusception - CDC discontinuing use. |
|
|
Rotavirus Vaccine: RotaTeq®
Clinical study: 11 countries (3 continents); 72,324 infants – 6 cases intussusception in RotaTeq® group – 5 cases in placebo group |
Live, oral, pentavalent (G1, G2, G3, G4 & P8)
3 doses: 6-32 weeks of age – 1st dose between 6-12 weeks of age, - 3rd dose NOT given after 32 weeks of age – US $63.96/dose |
|
|
Rotavirus Vaccine: Rotarix®
• Clinical study – Latin America & Finland: 63,225 infants – 6 cases intussusception Rotarix® group – 7 cases in placebo group |
Live, attenuated oral, G1P8
• 2 doses – 1st 2 months of age – 2nd 4 months of age – US $92.15/dose (2) |
|
|
Norwalk virus (Norovirus): Family Calciviridae (2002, Noroviridae)
4 Genera (Norovirus, Sapovirus, Lagovirus & Vesivirus) |
Diarrhoea stool specimens from gastroenteritis patients
Small (27nm dia), Non-enveloped, Amorphous surface: feathery, ragged outline Virion: ss +ve sense RNA (7.5kb), Single structural protein (60 kDa) |
|
|
Norwalk-like viruses: Sapoviruses
• Considerable genetic homology with Norwalk • Shared virological characteristics |
Family Calciviridae
• Children & Adults (Elderly): 5 genogroups (GI-GV), Humans I II IV & V - 66% gastroenteritis outbreaks in Long term Care facilities |
|
|
Sapoviruses: Epidemiology & Pathogenesis
- – Winter Vomiting Disease - Transmission: 1 Faecal-oral route, Water-borne, Food-borne (raw shellfish) |
Estimated 50% outbreaks of acute, nonbacterial gastroenteritis (US)
Older children & adults (Children <5yrs • Virus multiplies in small intestine • Produces transient lesions of intestinal mucosa • Spares large intestine (NO faecal leukocytes) • Shed in faeces |
|
|
Sapoviruses: Clinical & Diagnosis
- Mild & Brief (Fatalities are RARE): 24-48hr following ingestion, Lasts 24-60hrs |
abdominal cramps, myalgias, malaise, headache, nausea, low grade fever & 1-2 days diarrhoea
Diagnosis (not sensitive) – 1) Mean (or median) illness duration of 12-60 hrs – 2) Mean (or median) incubation period of 24-48 hrs – - 3) > 50% of people with vomiting – 4) No bacterial agent previously found |
|
|
Sapoviruses: Detection
- Virus in stool (peak at day 2/5 after onset) • Difficult to culture, RT-qPCR assays, High Sensitivity (10-100 virus copies/reaction), RT-PCR (for genotyping), EIA (not sensitive) |
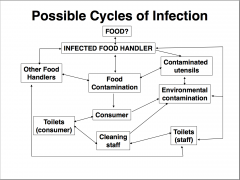
Cruise Ship Outbreaks - sanitisation to cleaning & disinfection
|
|
|
Norovirus Vaccine
2 doses: 3 weeks apart – IM 47% efficacy against illness – Illness less severe, shorter duration – No observed side effects |
LigoCyte® Pharmaceuticals Inc.
– norovirus virus like particle (VLP) vaccine (with chitosan and monophosphoryl lipid A as adjuvants) |
|
|
Adenoviruses: Adenoviridae
- 2 Genera: Mastadenovirses & Aviadenoviruses - Small 70-100nm dia, Icosahedral protein shell, 252 capsomeres, Protein core: ds DNA, 12 vertices PENTONS each with fibre, 2 serotypes associated: 40 & 41 (Group F) |
Pathogenesis: Respiratory Tract
- Infect: Epithelial cells of Pharynx, Conjunctiva, Small Intestine & occasionally other organ systems Spread beyond regional lymph nodes NOT usual • Replicate in intestine & present in stool • Diarrhoea with or without vomiting • 5-15% cases diarrhoea (2o Rotavirus) |
|
|
Astroviruses: Diarrhoea in Scotland
• Family Astroviridae • Small 27-30nm dia • Non-enveloped • Smooth or slightly indented outer shell • Inner 5 or 6 pointed star shaped core • 6.8kb ss +ve sense RNA |
• 2-8% sporadic cases in infants (3o Rotavirus)
• Infection through year: Peak in winter • 7 human serotypes UK: Type 1 prevalent (65% cases) Mexico: 6% cases, Type 2 prevalent (31%) Japan: Type 6 |
|
|
Small Round Structured Viruses
Toroviruses: Emerging GI Pathogen (similar to coronavirus) • At risk?: Aged, immunosuppressed & hospitalised |
Status uncertain
• Some bona fide Calciviruses or Astroviruses • Require more molecular data |
|
|
Hepatovirus: Hepatitis A virus (HAV)
• 27nm icoshedral particle (Picornaviridae structure) • Non enveloped symmetrical ss (+ve) RNA • 1983 Enterovirus 72: Biochemical & Biophysical - Virus shedding in faeces (10-14d after exposure) |
Spread: Faecal-Oral route, person-to-person Poor sanitation & overcrowding (strawberrys, clams, etc)
- Frozen Organic Antioxidant Blend Products |
|
|
Hepatitis E Virus (HEV)
• 32-34nm icoshedral particle (similar Calciviruses) • Non enveloped symmetrical (+ve) RNA • Final taxonomic classification? |
Enterically-transmitted non-A, non-B hepatitis
• Incubation longer than HAV (mean 6 wks) |
|
|
Inflammatory Diarrhoea
|
– Shigella spp.
– E. coli (EIEC) – Salmonella Serotypes – Campylobacter spp. – Yersinia spp. – V. parahaemolyticus & V. vulnificus – EAEC & STEC (EHEC) (Non invasive organisms - cytotoxin) |
|
|
Inflammatory Diarrhoea
Food associated: foodborne infection - Ingestion of organisms present in food - Colonisation & Invasion of intestines (Except EAEC & EHEC) |
Symptomology: Bloody diarrhoea (May begin as watery diarrhoea)
Longer incubation due to colonisation Fever may be present Toxins Affect: Enterotoxins &/or Cytotoxins |
|
|
Inflammatory Diarrhoea: Shigella sp.
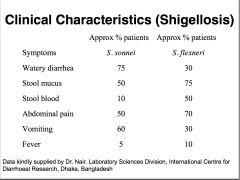
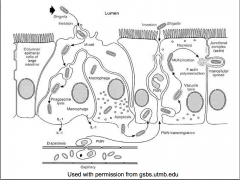
Genus: 50 species, into 4 groups “O” antigens Virulence factors: • Endotoxin(Oantigen) • Exotoxin: Enterotoxin acts as neurotoxin Causes: meningismus & coma, ulceration • NAD glycohydrolase Destroys all NAD in human cells Shuts down metabolismm Cell death |
Closely related to E. coli (antigens & toxin-capabilities))
GROUP A: Shigella dysenteriae GROUP B: Shigella flexneri GROUP C: Shigella boydii GROUP D: Shigella sonnei |
|
|
|
|
|
Bacillary Dysentery: Shigella dysenteriae type 1 (Shiga bacillus)
|
Shiga toxin (cytotoxin) - inhibits protein synthesis
Enterotoxin - produces diarrhea Exotoxin - inhibits sugar & Aa absorption in SI Neurotoxin - affects CNS |
|
|
Shigellosis by other Shigella sp.
= Readily transmitted faecal-oral route: Sanitation breaks down (4 F’s) • Bloodstream invasion RARE |
• Shigella sonnei - children <5 years (day-care)
• Shigella flexneri – men who have sex with men • Shigella boydii - rare Management: Antibiotics: chloramphenicol, ampicillin, tetracycline most common |
|
|
Shigellosis by other Shigella sp.
- Diagnosis, Isolation & Identification • Isolation from stools, water & food - MacConkey agar pale/colourless colonies - S-S agar (Salmonella-Shigella agar) |
Non-motile
Gram-ve rod NO fermentation lactose NO utilization citric acid NO H2S production (except. S. flexneri) NO gas from glucose |
|
|
ENTEROINVASIVE E. coli (EIEC)
SE Asia/S America Similar to Shigellosis less severe (Often mistaken) NO Shiga toxin Infective dose as few as 10 organisms |
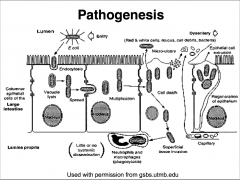
• Pathogenesis: Invasion of enterocytes in LARGE INTESTINE, Inhibits protein synthesis, killing host cells
Causes dead WBC’s (pus), RBC’s and mucosal cells in stool • Management Rehydration therapy |
|
|
Salmonella Genus: • Ubiquitous pathogens
2200 different types based on Vi antigens (Capsular) Salmonella enterica subspecies enterica serotype XXX |
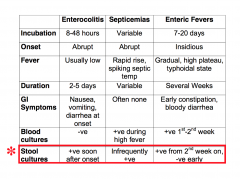
• Salmonellosis: Clinical Manifestations (3 Types)
• Gastroenteritis S. Typhimurium, S. Enteritidis, S. Newport • Septicemia/Bacteremia(focalinfection)Rare S. Cholerasuis • Enteric (Typhoid) Fever S. Typhi |
|
|
|
|
|
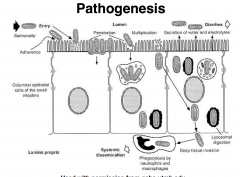
Enterocolitis (Gastroenteritis)
• Salmonella “food-borne infection” Localized infection, Excessive fluid secretion from ileum & jejunum |
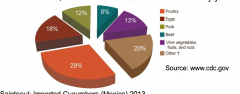
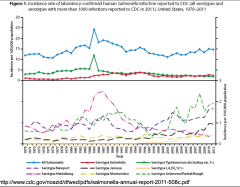
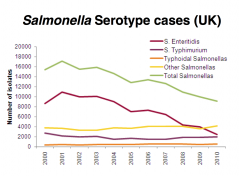
Salmonella Heidelburg: October 2013 - March 2014
Foster Farms Chicken |
|
|
|
|
|
Reptile-Associated Salmonellosis
8 Outbreaks: 473 individuals, 29% hospitalisations, 71% <10yo |
• Infants/children: direct or indirect contact
• Lizards, snakes, turtles & frogs Turtles <4inches banned in US (1975) 77% redn in turtle-associated Salmonellosis |
|
|
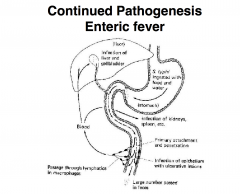
Enteric Fever: S. Typhi
Important morbidity/mortality worldwide US: ONLY seen in traveller’s to Asia, Mexico, India TYPHOID FEVER Enteric fever: S. Paratyphi A, B or C |
|
|
|
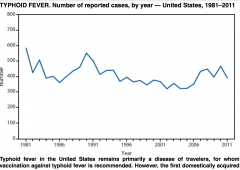
Enteric Fever: ASYMPTOMATIC CARRIAGE
2-5% typhoid patients - excrete 1-1,000 mill. S. Typhi/g faeces Carrier state important in transmission |
• Management: Chloramphenicol/Ciprofloxacin
• Prevention: 3 Developed vaccines (See CDC website) |
|
|
Outbreak: Salmonella Typhi
• Frozen Mamey Fruit Pulp Motile Gram-ve rod NO fermentation lactose H2S production Gas from glucose Serotyping |
Diagnosis, Isolation & Identification
Isolation from stools, water & food MacConkey agar pale/colourless colonies S-S agar (Salmonella-Shigella agar) |
|
|
Diagnosis of S. Typhi
|
1) History of travel to endemic areas
2) Rose coloured spots on abdomen (2-4days) 3) Examination of blood (anaemia, leukopenia, absence of eosinophils) 4) Isolation of S. Typhi on S-S agar 5) Positive Widal reaction (agglutination of O & H antigens) |
|
|
Campylobacter species: exclusively veterinary disease, but common cause of diarrhea in humans
– C. jejuni (subsp. jejuni, doylei), C. coli – C.lari,C.hyointestinalis |
Small, curved-spiral rods
1.5 - 3.5 mm long by 0.2 - 0.4mm wide Gram -ve Non-sporing Motile (single polar flagellum) Microaerophilic - req. 5% O2, 10% CO2 for growth DO NOT ferment CH2O Catalase +ve No growth at 25oC, but at 37oC, readily 42 - 43oC |
|
|
Campylobacter species: EPIDEMIOLOGY
Intestinal tract of wide variety of wild & domestic animals (Zoonotic) • Faecal contaminated water |
Commercially raised poultry
Normal commensal of cows Long-term commensal of sheep Pigs (carriers of C. coli) C. jejuni intestinal commensal Cats & dogs |
|
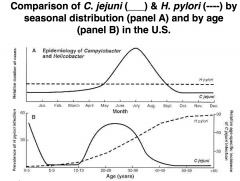
|
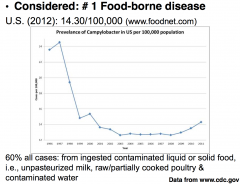
60% all cases: from ingested contaminated liquid or solid food,
i.e., unpasteurized milk, raw/partially cooked poultry & contaminated water |
|
|
Campylobacter species:PATHOGENESIS
• Dose - 104 org (as few as 500 cells) INVASION: Inflammation & Bacteremia TOXIN: – Endotoxin – Enterotoxin: watery diarrhoea – Cytotoxin: Verotoxin similar to Shiga toxin: (Haem colitis) Significance not understood |
CLINICAL• Symptoms: 3-5 days after ingestion
Vomiting - slight Diarrhea - often profuse (green?) Abdominal pain - often severe Prostration - often severe Pyrexia - often present Other symptoms - bloodstained faeces |
|
|
Campylobacter species: Management
Usually self-limiting Erythromycin - eradicate C. jejuni from faeces Severe abdominal pain - aminoglycoside, chloramphenicol, doxycycline |
Association/Complications
– Reactive arthritis (1% cases): Knee joint (6-12 mnths) – Acute Inflammatory Demyelinating Polyneuropathy: Guillain-Barré syndrome (GBS) (30% cases) AKA: Acute Motor Axonal Neuropathy |
|
|
Detection of C. jejuni
|
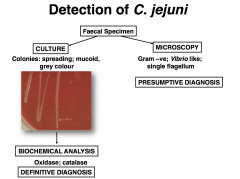
|
|
|
Yersinia enterocolytica (Yersiniosis)
= Lesser cause Y. pseudotuberculosis • Common in children <7 yrs (1-4 y); adults • Rivals Salmonella - acute gastroenteritis (cooler climates) • -1 - +40oC (Psychrotroph – Facultative psychrophile) |
Pathogenesis (Poorly Understood)
• Invasive induces inflammatory response Distal ileum (gut-associated lymphoid tissue) Adjacent tissues & mesenteric lymph nodes also infected (mimic appendicitis) • (Chromosomal) ST Enterotoxin • inc cGMP |
|
|
Yersinia enterocolytica (Yersiniosis)
- CLINICAL FEATURES • Management: oxytetracycline or doxycycline |
Self-limiting enterocolitis
Incubation period 3-7 days Lasts 14-21 days (Longer) Symptoms: abdominal pain & diarrhoea Mild fever, vomiting rare |
|
|
Post-infective Reactive Arthritis (Autoimmunity Arthritis)
• Small proportion patients (poorly understood) – Induced polyclonal T-cell stimulation (toxin) – Non-specific immune stimulation of invasin binding to b1 integrins on T lymphocyte – Other bacterial antigens |
Diagnosis, Isolation & Identification
Special investigation • Diagnosis from stool is possible Often considered late: rising antibody titres in paired serum • MacConkey (pinpointcolonies/48hrs) • Specialized Yersiniamedia |
|
|
NON - CHOLERA Vibrio’s
- common along the gulf coast |
Not agglutinated by anti O1 sera
Halophilic organisms (Common coastal waters) V. parahaemolyticus V. alginolyticus V. vulnificus V. cholerae (NOT toxigenic V. cholerae O1 or O139) |
|
|
Vibrio parahaemolyticus
• Ingestion of raw/poorly cooked seafood Acute abdominal pain, vomiting & watery diarrhea Japan - raw fish (# 1 Food-borne) US - shellfish • Treatment: tetracycline |
• May-October 2013
Clams & Oysters, Harvest Area Closures |
|
|
Vibrio vulnificus - Diarrhoea & infection of cuts (Salt water abrasions) -- Vibrio Illnesses after Hurricane Katrina --- Multiple States
Virulent strain: Management: tetracycline Intense skin lesions (gastroenteritis & even severe bacteremia) |
Clinical presentation (Not Cholera)
Screening of stool samples Oxidase activity Thiosulphate-citrate-bile salts-sucrose (TCBS) agar Sucrose (differentiating agent) Sucrose -ve V. parahaemolyticus, V. vulnificus |
|
|
ENTEROAGGREGATIVE (EAEC)
• PATHOGENESIS: Not fully understood NO EAF (Enteric Adherence factor) Possess AAF (Aggregative Adherence factor) |
1) Initial adherence to intestinal mucosa and/or mucus layer (fimbriae)
2) Enhanced mucus prodnthick mucous biofilm 3) Cytotoxin prodn?damage to intestinal cells |
|
|
ENTEROHAEMORRHAGIC E. coli VEROTOXIN PRODUCING (VTEC)
SHIGA TOXIN PRODUCING (STEC) causes: LIFE THREATENING CONDITIONS |
HAEMORRHAGIC COLITIS
HAEMOLYTIC UREMIC SYNDROME (8 -11% cases) Acute renal failure, Thrombocytopenia, Microangiopathic haemolytic anaemia THROMBOTIC THROMBOCYTOPENIA PURPURA |
|
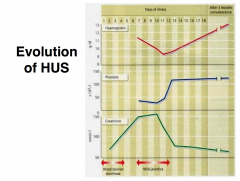
|
Pathogenesis: Attachment (similar to EPEC)
Phage encoded: CYTOTOXIN - VEROTOXIN - 2 types (VT1 & VT2) both AB toxins - Shiga-like toxin (rRNA) blocks protein synthesis |
|
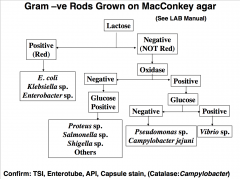
Diagnosis of E. coli’s: Routine stool culture
|
1) MacConkey’s agar (Red-Pink colonies)
2) Sorbitol MacConkey’s agar (no fermentation STEC) 3) (ETEC) Inoculate mouse adrenal cells: stimulation of adenylate cyclase by LT/ST 4) ELISA on toxin bound to antibody 5) DNA probe to detect toxin genes |
|
|
Other GI Tract Infections
|
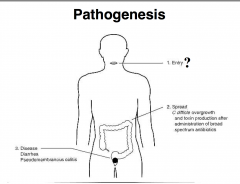
Antibiotic-Associated Diarrhoea - C. difficile
Gastritis/Duodenal/Gastric Ulcers - H. pylori Gastrointestinal Abscess (Peritonitis, Appendicitis & Diverticulitis) - E. coli & Bacteroides spp. + others |
|
|
Clostridium difficile
• NOT Food-borne • Normal flora 3% adults • MajorNOSOCOMIALpathogen • Spectrum of intestinal diseases |
• Antibiotic-Associated Diarrhoea
Usually cause only minor concern Can evolve into a life-threatening enterocolitis • Antibiotics Ampicillin, cephalosporins, clindamycin & amoxicillin • Induced Pseudomembranous Colitis Antineoplastic agents: methotrexate |
|
Clostridium difficile
|
2 toxins
Toxin A enterotoxin (fluid accumulation in bowel) (weak cytotoxin most mammalian cells) Toxin B potent cytotoxin Decreases cellular protein synthesis & disrupts microfilament system of cells (similar to diphtheria toxin) |
|
|
Clostridium difficile: Clinical Symptoms
• Vary: mild diarrhoea → severe abdominal pain accompanied fever (>101oF) & severe weakness • Diarrhoea: watery usually non-bloody (5-10% bloody) excess mucus & pus (or blood) Hypoalbumineia & Leukocytosis common |
Diagnosis: Difficult, Not distinguished from Ulcerative colitis & Crohn’s
Colonic examination (presence of pseudomembrane) AND isolation C. difficile, associated antibiotic therapy Toxin presence |
|
|
Pseudomembranous Colitis: Management
Discontinue antibiotic - symptoms resolve 1-14 days |
If severe or no response; treat oral antibiotics:
• Vancomycin (“gold” standard) or Metronidazole (milder infections) - Relapses in 15-20% patients • Dificid (fidaxomicin) bid 10 days (US $2,800 for 10d course) • Faecal microbiota transplantation |
|
|
Helicobacter pylori: 1982 (Australia) from gastric biopsies Named Campylobacter pyloridis (C. pylori)
1989 Helicobacter pylori Biological carcinogen |
MORPHOLOGY
Gram -ve Non spore-forming Curved to spiral (1-3 turns) Motile - polar (5-6) flagella Microaerophilic 2-5%O2, 5-10%CO2 Catalase +ve; Urease +ve Coccoidal forms under culture |
|
|
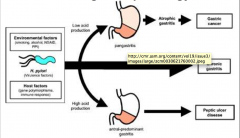
H Pylori: ASSOCIATED CAUSE
• Gastritis (stomach atrum) • Duodenal ulcers (& gastric ulcers) • Gastric cancer |
Factors contributing to H. Pylori related gastric pathology
|
|
|
H Pylori: Pathogenesis
• Gastric colonisation is common • Route of infection UNCLEAR • Role of cytotoxin, urease, mucinase, flagella mechanisms UNDER INVESTIGATION |
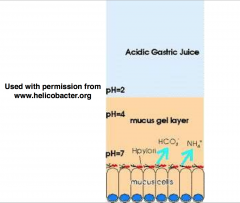
• UREASE allow H. pylori survival at pH 2.0
Able to split ammonia from urea = alkaline environment • Virulence Factors: allowing for adhesion & damage to mucosa - cagPAI: VacA cytotoxin; BabA; OipA |
|
|
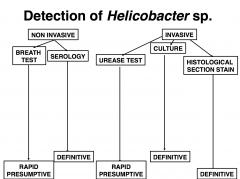
|
|
|
H. Pylori Management
• H. pylori sensitive in vitro DO NOT WORK Monotherapy in vivo • EffectiveTherapies – Triple, Quadruple or Sequential regimens |
• Triple Therapy (clarithromycin resistance <15%)
– PPI (lansoprazole, omeprazole, pantoprozaole or esomeprazole) + amoxicillin (bid), and clarithromycin (bid) – 7-14 days • Quadruple Therapy (clarithromycin resistance ≥ 15%) – PPI, combined with bismuth (qid) + two antibiotics (e.g., metronidazole (qid) and tetracycline qid) – 10-14 days |
|
|
Helicobacter resistance to clarithromycin in different countries
Vaccination? - Helivax Inactivated, whole cell vaccine |
Initial Vaccine Studies:
Mice vaccinated - Oral vaccine H. felis sonicate + mucosal adjuvant cholera toxin (LT E. coli) = complete protection |
|
|
H. Pylori: Gastrointestinal Abscess
• Peritonitis • Appendicitis & Diverticulitis • Intra-abdominalabscess • Liver abscess • Pancreas abscess |
healthy GI tract >300 bacterial species
Pathogenesis • Reduced O2 tension & Oxidation-reduction potential • Impaired blood supply; necrosis of tissue, growth of facultative anaerobes |
|
|
H. Pylori
• Association: vascular disease, trauma, surgery, presence of foreign bodies, malignancy, radiation therapy, injection of vasoconstrictive agents, shock, cold or oedema • Provides: anaerobic environment, impaired host defenses • Enable opportunists to grow |
Clinical Symptoms & Causes
- E. coli. Also by tuberculosis, N. gonorrhoeae & C. trachomatis infections Peritonitis: pain, abdominal distention, diffuse muscle spasm, tenderness & rebound tenderness, decreased/ absent peristalsis, rigidity of abdominal wall, tenderness on rectal or vaginal exam, fever & leukocytosis 1st peritonitis (spontaneous bacterial peritonitis) 2nd peritonitis from spillage of bacteria |
|
|
H. Pylori: Appendicitis:
(See pseudoappendicitis: Y. enterocolytica) • Pelvic abscess: pain, deep tenderness in 1 or both lower quadrants, fever, urinary frequency, dysuria & diarrhoea with passage of mucus in the first stools. Rectal or vaginal examination may reveal tenderness |
H. Pylori • Diverticulitis: Abdominal pain, peritoneal irritation, fever & leukocytosis
– Acute diverticulitus (peridiverticulitis) – Microperforations of diverticula cause contamination of peritoneal cavity by aerobic and anaerobic flora resident to normal colon – Most Common Causes: Bacteroides spp., E. coli & enterococci |
|
|
H. Pylori
Pylephlebitis & Liver abscess: Chills, fever, epigastric or right upper quadrant pain, nausea, vomiting, enlargement & tenderness of liver • Pancreatic abscess: (acute) severe epigastric pain. Recent history of excessive ingestion of food & alcohol. Nausea & vomiting are common |
– Cholecystitis: obstruction of cystic duct with subsequent bacterial invasion of the gall bladder
– Most Common Causes: E. coli, Klebsiella sp., Enterobacter sp., Proteus sp., P. aeruginosa, enterococci, streptococci, staphylococci, Bacteroides sp., Clostridium sp., Fusobacterium sp., peptostreptococci |
|
|
H. Pylori Diagnosis:
– Type and location of pain – WBC count – Biochemistries – Imaging (X-ray, computerised tomography, position emission tomography, ultrasound & radioisotope imaging - gallium or indium) – Pathognomonic signs |
Treatment Principles:
Improve vascular perfusion (correct fluid & electrolytes) Combat effects of bacteria & toxic metabolites Reduce paralytic ileus Eliminate primary source of infection Aspirate infected exudate (drain primary lesion) Treat local or distant complications |
|
|
Antimicrobial Therapy: Anaerobes resistant to penicillins, cephalosporins & most amino glycosides
Possibilities: Chloramphenicol (succinate): Bacteroides fragilis Metranidazole: All Bacteroides spp. Gentamycin, Tobramycin & Amikacin: useful Clindamycin: 60% Bacteroides spp sensitive |
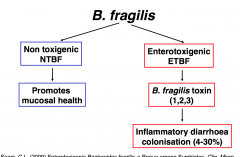
|
|
|
Management of Diarrhoeal Disease
Oral rehydration (ORT): till normal rehydration restored (determined by: clinical condition/body weight) Sodium: 150-155mmol/l Glucose: 200-220mmol/l Potassium: 4-5mmol/l |
Intravenous rehydration: shock, exhaustion precluding oral feeding and oral rehydration failure
Antiemetic drugs: reduce fluid loss oral rehydration effective Antidiarrhoeal drugs: RARELY SUCCESSFUL (reduce gut motility - allow accumulation of fluid) |
|
|
Management of Food-borne Disease in the Community
• Developing countries: Poor water quality GI infections: waterborne • Developed countries: Food important source Ideally: free from pathogenic bacteria In practice: frequently contaminated |
Prevention of Infections
• Safe food production: healthy flocks & herds, avoid sewage to fertilize crops • Food manufacture processes: hygienic slaughter & meat packing, rodent-free storage of crops, store at chill or refrigeration temps, hygienic packaging, cold chain during distribution |
|
|
• Domestic & commercial food hygiene: adequate refrigeration, avoidance of cross-contamination, usage within spoilage dates, adequate decontamination of food by washing and/or cooking, personal & kitchen hygiene
|
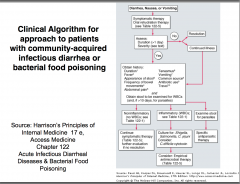
|

