![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
DNA is ? To RNA is ? To Protien This is the what? |
1) transcribed 2)translated 3) Central Dogma of Molecular Biology |
|
|
What is DNA? |
Genetic Material. The molecule that is packaged into chromosomes and is passed from one generation to the next during cell division and sexual reproduction. |
|
|
What is DNA made of? |
Polymers. Which are made up of atoms of carbon, hydrogen,nitrogen,oxygen,and phosphorus found in a repeated arrangement of interconnected, small molecules. |
|
|
Which macromolecule is DNA? What does that mm do? |
Nucleic acid. Carries info to produce specific protiens and regulate cellular activities. |
|
|
DNA and RNA are made up of what chain of monomers? |
Nucleotides. |
|
|
The 5 bases for RNA and DNA |
Cytosine (C), Guanine (G), Adenine (A), Thymine (T), (For RNA only uracil (U)) |
|
|
What does Adenine look like? Details? Top letter thingy? How many hexagons? |
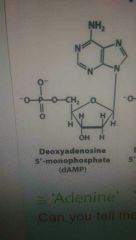
NH^2 and two hexagons |
|
|
What does guanine look like? Letter thingy at top? Hexagons? |
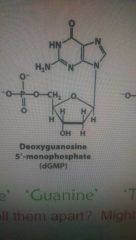
O two hexagon things. |
|
|
What does Thymine look like? |
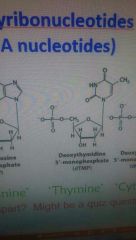
O and one hexagon thingy. |
|
|
What does Cytosine look like? |
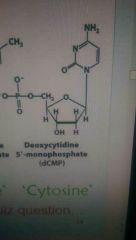
NH2 one hexagon thingy. |
|
|
What is dehydration synthesis? |
Removal of water= joining a new monomer to a chain. |
|
|
What is hydrolysis? |
Polymers are broken down to monomers with the addition of water. |
|
|
Nucleotides are linked together via ? to form long chains (?) |
Phosphodiester bonds and polynucleotides |
|
|
DNA has two ends; what are they? |
5' and 3' (5 prime and 3 prime) |
|
|
Nucleotides are assembled into chains called ? |
polynucleotides |
|
|
DNA has ? backbones ? interact in the middle it is anti? |
sugar-phosphate backbones bases interact in middle antiparallel |
|
|
? bonds with? purine: pyridimine: DNA is held together by ? |
A bonds with T (U) G bonds with C Purine: A and G Pyridimine: T and C and U hydrogen bonds |
|
|
Double helix can adopt different forms in different conditions. B- form: A- form: Z- form: |
B-form: most common under physiological conditions (lots of water) A form if less water Z form (left hand twist) may form under certain chemical conditions in the cell, more rare. |
|
|
RNA has ? strands? DNA encodes info to produce ? and ?; RNA helps make ? and regulate other info. |
1 RNA and Protiens Protiens |
|
|
Genes contain info in the form of ? that is used to create ? molecules, several diff. kinds of which are used to manufacture ? |
DNA RNA protiens |
|
|
Protein functions? |
Enzyme action Defense (immune function) Transport Support Motion Regulation (hormones) Storage |
|
|
Protein's monomer? polymer? Amount of amino acid variants? |
Amino acid polypeptide 20 each with a different R group |
|
|
A Side chain ( r group) is at the top of the protein. Nonpolar side chains = Polor side chains= Electrically charged side chains = |
hydrophobic hydrophilic hydrophilic |
|
|
Amino acids are joined together by ? which creates a ? |
dehydration synthesis peptide bond |
|
|
Each protein gains specific functionality by folding into a unique ?, which is determined by the specific ? they contain |
3-D shape amino acids |
|
|
Primary Structure? |
Specific number and order of amino acids. |
|
|
Secondary Structure? |
Regions of localized folding or coiling stabilized by hydrogen bonding between amino acid "backbones" (not R groups) |
|
|
Tertiary Structure? |
Overall 3D shape stabilized by various bonds between R groups of amino acids. |
|
|
Quaternary structure? |
More than one polypeptide that together create a functional protein ( this level is not required in all proteins) |
|
|
Protein unfolding: Denaturation |
Protein loses structure and function due to environmental conditions. - pH -Temperature - Ionic concentration of solution (add salt/ water to change) |
|
|
Why does denaturing matter? Fevers? What is a fever? What can it indicate? When do human proteins begin to denature? |
Fever is the body's intentional temperature increase to inhibit ideal bacterial growth conditions and disrupt bacterial proteins. High/persistent fever can indicate underlying medical emergencies. 106- 107 degrees Fahrenheit |

