![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
65 Cards in this Set
- Front
- Back
|
Describe the Bohr model of the atom
What makes up the nucleus? Emitted and Absorbed energy directions? |
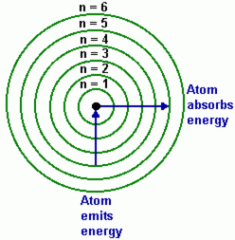
The nucleus is made up of protons and neutrons and the e-'s are found within the e- cloud
|
|

1) Describe what the 12 signifies and what the 6 signifies.
2) Explain how e-'s come into play? 3) What is an isotope? |
1) The 12 is the mass #, which is protons + neutrons
The 6 is the atomic number, which never changes unless the element changes. This is only protons. 2) The e-'s = the amt of protons in the neutral state 3) Another variation of an element that differs by weight: 12C, 13C, 14C |
|
|
1) How much does a proton weigh?
2) How much does a neutron weigh? 3) How much does an electron weigh? |
1) 1.67 x 10^-27 kg
2) 1.67 x 10^-27 kg 3) very close to 0 |
|
|
1) Which is the prevalent form of Cl? 35Cl or 37Cl
2) How much more prevalent is that form? atomic mass: 35.5 g/mol |
1) 35Cl
2) 75% more prevalent (3:1 ratio) |
|
|
What are the 5 major groups you should know from the periodic table?
Describe their reactivity |
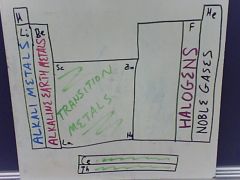
Alkali Metals: Form very strong bases
Alkaline Earth Metals: React very strongly with H2O Transition Metals: Often form colored compounds Halogens: Reactivity increases as you go up this column, F is the most reactive Noble Gases: Not very reactive |
|
|
Describe the location of metals and nonmetals
|

Metals tot he left of staircase, nonmetals to the right
|
|
|
Describe the amount of orbitals for s, p, and d
How many e-'s can be held by s, p, and d? |
s --> 1 & 2e-
p --> 3 & 6e- d --> 5 & 10e- |
|
|
1) Paramagnetic?
2) Diamagnetic? |
1) Ion/element attracted to a magnetic field (unpaired e-)
2) Slight deflection away from magnetic field (all paired e-) |
|
|
How many valence electrons are present?
- S[Ne]3s^2 3p^4 -Cr[Ar]4s^1 3d^5 -Br[Ar]4s^2 3d^10 4p^5 -Cu[Ar]4s^1 3d^10 |
-6
-6 -7(remember once the d orbital is filled it doesn't count) -1ish |
|
|
Explain the significance of the quantum numbers:
n --> l --> m(l) --> m(s) --> |
![n = principal quantum #/shell # [1....infinity]
l = Azimuthal #, the subshell (s,p,d,etc) 0=s, 1=p, 2=d [0...(n-1)]
m(l) = Magnetic # - specific order [-l ...+l]
m(s) = spin# [+1/2 or - 1/2]](https://images.cram.com/images/upload-flashcards/853129/2401816_m.png)
n = principal quantum #/shell # [1....infinity]
l = Azimuthal #, the subshell (s,p,d,etc) 0=s, 1=p, 2=d [0...(n-1)] m(l) = Magnetic # - specific order [-l ...+l] m(s) = spin# [+1/2 or - 1/2] |
|
|
What is the strong nuclear force/nuclear binding energy?
|
The force that holds protons together within the nucleus even though they repel one another.
|
|
|
Describe the 5 routes of nuclear decay
|

Make real index card of this, a problem, and the graph
|
|
|
What are two main characteristics of a stable nucleus?
|
1) Even # of neutrons and/or protons
2) N/Z =1for Z<20, the closer to 1 the better |
|
|
1) The rate at which decay occurs is proportional to _____
2) Solve this problem: If the 1/2 life is 10yrs of a 100g substance, how much remains after 40 years? |
1) how much you have
2) 6.25g |
|
|
1) Why does a nucleus always weigh less than it's separate parts?
2) What is the most stable nucleus in the universe? WHat can we determine based on this? |
1) Some mass turns into energy, E=mc^2
2) 56_26Fe the closer atoms are to this atom, the higher nuclear bonding energy they have (Ex: 54C vs 238U --> 54C is higher) |
|
|
_Periodic Trends_
1) Atomic radius (Ex) 2) Ionic radius (Ex) 3) Electronegativity (Def) 4) Ionization Energy (Def) 5) Electron Affinity (Def) |
1) Smaller towards F (Ex. Mg has 1 more e- in the valence shell than Na and therefore feels a stronger attraction towards the nucleus)
2) Ex. --> Mg+2 is smaller b/c it looses an entire valence shell in comparison to Mg. The more positive the smaller; the more negative the larger 3) Increases towards F (how tightly an atom holds onto e-'s) 4) Increases towards He (E required to remove an e-) 5) Becomes more negative/exothermic to the right (E change when gaining an e-) |
|
|
Types of bonds:
1) Covalent 2) Ionic 3) Metallic |
1) non-metal & non-metal
-covalent network: diamond(C) & quartz(SiO2) -Molecular-forms molecules 2) non-metal & metal (salt) -can't move or conduct electricity unless in liquid -high m.p. & b.p. 3) metal & metal -conductive (ex. metal bench) -ductile (can be easily drawn into wires) -malleable (can be pounded into sheets) -luster (shine) |
|
|
Compare Lewis, Arrhenius, and Bronsted-Lowry acids and bases
|
Lewis - (covers all acids & bases) base: e- donor, acid: e- acceptor
Bronsted-Lowry - (the second best) base: H+ acceptor, acid: H+ donor Arrhenius - (worst) base: H+ acceptor in H2O, acid: H+ donor in H2O |
|
|
Difference between ionic and covalent bonds
|

Ionic: Atom gives away electron, both become ions, the + and - charges attract (metal and nonmetal)
Covalent: Electrons are shared between two atoms (nonmetal and nonmetal) |
|
|
Describe the electron domain geometry and angle value for the 5 most popular types
|

Linear 180
Trigonal Planar 120 Tetrahedral 109.5 Trigonal Bipyramidal 90 & 180 & 120 Octahedral 90 |
|
|
What is the electron domain geometry and molecular geometry for NH3?
H2O? |
EDG: Tetrahedral
MG: trigonal pyramidal EDG: Tetrahedral MG: Bent |
|
|
For the e- domains, what would the hybrid of the orbitals be?
2: 4: 6: |
2: sp
4: sp3 6: sp3d2 |
|
|
Describe polar vs non-polar bonds
Are these polar or non-polar? 1) HCl 2) Cl2 3) CO2 4) BF3 5) NF3 |
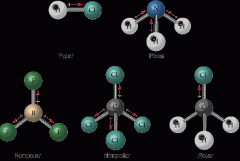
If the dipoles within the molecule cancel each other out, it is non-polar. If they dont, it's polar
1) polar 2) nonpolar 3) nonpolar 4) nonpolar 5) polar |
|
|
Intermolecular Forces:
1) H-bonding 2) Dipole-Dipole 3) London Dispersion (Van der Waals) 4) Ion - dipole |
All are significantly weaker than ionic and covalent bonds
1) F-H, O-H, N-H --> strongest intermolecular force 2) H+-Cl-----H+-Cl- 3) Nonpolar molecules ONLY have london dispersion. Bigger molecules have more 4) Ex. - Na surrounded by water molecules, partial neg. on O attracted to + charge on Na -->close in strength to H-bond |
|
|
If a molecule has a lot of I.M. forces, it is:
1) Big/Small 2) has __M.P. 3) has __B.P. 4) has __vapor pressure 5) has __viscosity 6) has __surface tension |
1. big
2. higher 3. higher 4. lower 5. higher 6. higher |
|
|
1) Which has the highest B.P.?
a: CH4 b: CH3OH c: CH3F 2) Which has the highest B.P.? a: NaCl b: CH4 c: CH3OH d: CH3F |
1) B
2) A |
|
|
What is it called when:
1. solid --> liquid 2. liquid --> gas 3. solid --> gas 4. gas --> liquid 5. liquid --> solid 6. gas --> solid |
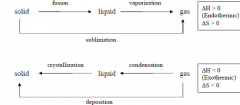
1. fusion
2. vaporization 3. sublimation 4. condensation 5. crystallization 6. deposition |
|
|
1. Which phase changes are endothermic?
2. What would the change in H and S be for endo? 3. Which phase changes are exothermic? 4. What would the change in H and S be for exo? |
1. fusion, vaporization, sublimation
2. ΔH > 0, ΔS > 0 3. condensation, crystallization, deposition 4. ΔH < 0, ΔS < 0 |
|
|
1. What is the change in H?
2. Describe Gibbs Free Energy 3. At eq, Gibbs = ? |
1. The change in Enthalpy - the amount of Energy within a system
2. G takes enthalpy and entropy into account in order to determine the spontaneity of a rxn. The formula for Gibbs is ΔG = ΔH - TΔS. A rxn is spontaneous when ΔG is negative 3. 0 |
|
|
Describe phase changes based on the addition of heat and the change in tempture.
What is the formula for heat? Does ΔT change for when switched from K to C? |
When heat is added at a temperature below the freezing point, the temperature increases until it reaches the phase change pt (solid to liquid). As heat increases, the temperature does not change during the phase change. When the phase change pt pases, temp increases again until it reaches the next phase change.....temp remains constat throughout, then increases again when past that pt
q = mcΔT (MCAT*) No |
|
|
Describe the phase diagram of CO2
Where is the triple pt, critical pt, and normal B.P. located? What is the B.P., triple point, and critical pt? |
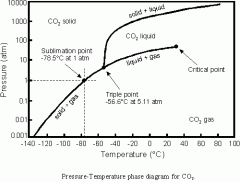
-Mention solid, liquid, gas locations and the axis
B.P. - Pt at which the vapor pressure of H2O equals the atmospheric pressure Triple Point - Pt at which all three phases exist Critical Point - Pt at which you cannot differentiate between liquid and gas |
|
|
What are the 2 ideal gas assumptions?
What is gas at STP? What 4 units (w/values) can P be converted into? |
a: No intermolecular attractions
b: Gases have no volume. They take up a lot of space, but the majority of the space is empty. (only at low P) P = 1atm, T = 273K, V = 22.4L for 1 mol of gas 1 atm = 760mmHg = 760torr = 100,000Pa |
|
|
1. What is the ideal gas law equation?
2. What is the combined gas law equation? 3. If gas is in a container with a piston and the piston is pushed down, decreasing the volume, P ___ , T ___ , & Intermolecular forces ___ 4. What is the value of R? |
1. PV = nRT
2. P1V1/n1T1 = P2V2/n2T2 3. P increases, T increases, & Intermolecular forces increase 4) 0.08206 L*atm/mol*K or 8.314 J/mol*K |
|
|
1. What is Avogadro's Law?
2. What is Boyle's Law? 3. What is Charle's Law? |
1. V is proportional to n, 6.02 x 10^23 particles w/in a mol
2. V is proportional to 1/P, As P increases - V decreases 3. V is proportional to T (Why? T is measured by how excited atoms are - as V decreases there is less space for the molecules to act in the excited state. Hence, as V decreases, T decreases) |
|
|
Equations for density and molar mass?
Describe Dalton's Law od Partial Pressures. |
d = m/V
mu = m/n Ptotal exerted by the mixture of non-reactive gases is equal to the sum of the partial pressures of individual gases: Pa + Pb + Pc..... = Ptotal Pa = Xa(mol fraction) * Ptotal |
|
|
Why, in Grahm's law of diffusion, does a lighter gas move at a higher velocity - equation based question?
|
Grahm's law explains how gases move through a narrow slit. The lighter gas moves faster because the K.E.'s of the gases are equal to one another and smaller mass divides to give a larger number.
Ex. 1/2mv^2 O2 = 1/2mv^2 H2 |
|
|
1. If you mix two liquids, how do you determine which is the solvent and which is the solute?
2. Saturated? 3. Unsaturated? 4. Supersaturated? |
1. The solvent is the more prevalent liquid
2. The max amt of solute has been added to the solution 3. The max amt of solute has not been added to the solution 4. More than the max amt of solute has been added to the solvent - this is done by using heat |
|
|
1. Molarity?
2. Molality? |
1. M = moles of solvent/Liters of solution (solvent & solute)
2. m = moles solute/kg of solvent (these two are similar in water b/c 1L = 1kg, but for other substances, this is not the same) (they differ the most when the concentration increases) |
|
|
What are 2 solubility rules?
|
1) Soluble = ALL group 1 metals, NO3-, NH4+, ClO4-, C2H3O2- salts
2) Insoluble = Most Ag+, Pb2+, Hg2+2 salts (unless they're with something from 1) |
|
|
1) Solids are more at ___________ temps
2) Gases are less soluble at _____________ temps 3) Gases are more soluble in a liquid at _________ pressure |
1) higher
2) higher 3) higher (higher pressure forces the gases down into the solution) |
|
|
1) What would make a freezing pt even lower?
2) What is the van't hoff factor for: a. NaCl b. Na2SO4 |
1) If irregularities are present.
2) a. i = 2 b. i = 3 |
|
|
1) Osmosis?
2) Osmotic Pressure? 3) Describe a basic reaction coordinate diagram |

1) The shift of water from high P to low P
2) The pressure it takes to keep water from entering |
|
|
1) Why do most chemical rxns slow down overtime?
2) What is the rate law equation for: 2NO(g) + Cl2(g) ---> 2NOCl(g) 3) The rate law is determined from the (slow/fast) rxn 4) What is a catalyst? |
1) Because it's rate is proportional to reactant conc.
2) Rate = k[NO]^2[Cl2] 3) slow 4) Speeds up a rxn by lowering the Ea by providing a diff. mechanism (pathway) for the rxn to occur |
|
|
1) What is the Arrhenius equation?
2) What is the definition of equilibrium? 3) If Keq >> 1, this means: 4) If Keq << 1, this means: 5) If Keq = 1, this means |
1) K = Ae^(-Ea/RT)
2) forward rate = reverse rate 3) there are more products than reactants at eq 4) more reactants than products at eq 5) similar amts of products and reactants at eq |
|
|
1) If Q > K, the result would be:
2) If Q < K, the result would be: 3) If Q = K, the result would be: |
1) shift left
2) shift right 3) at equilibrium |
|
|
1) What does the equilibrium constant & rxn quotient not include from a rxn?
2) What happened to Keq when we: a: reverse the rxn b: multiply everything by 3 |
1) Solids & liquids
2) a: inverts it 1/Keq b: cubes it |
|
|
Le Chateliers Principle Example:
C(s) + O2(g) <---> 2CO(g) 1) If O2 is added to the rxn, what happens? 2) If C is added, what happens? 3) If CO is removed, what happens? 4) If P increases, what happens? 5) If the inert gas Ar is initially added to the rxn, what happens? 6) What is the only thing that changes K? |
1) shifts right
2) nothing 3) shifts right 4) shifts left, shifts to sode with less moles of gas 5) the pressure increases due to more molecules present, but doesn't change the moles or V of the other species. There's no shift - doesn't participate in rxn 6) T. When concentration changes, Q changes, but not K |
|
|
What are 7 strong acids?
|
H2SO4
HClO4 HI HBr HCl HNO3 HClO3 |
|
|
What are the strong bases?
|
Group 1 metal hydroxides
Ba(OH)2 Sr(OH)2 Ca(OH)2 |
|
|
1) Describe the binary acid trend
2) What are two oxoacid trends? |
1) Acidity increases to the right and down (larger bonds have weaker bonds and are stronger acids)
2) the more oxygens, the more acidic --- the more electronegative heteroatom, the more acidic |
|
|
What are the equation for pH, pOH, H+, OH-?
|
pH = -log[H+]
pOH = -log[OH] [H+] = 10^-pH [OH] = 10^-pOH pH + pOH = 14 [H+][OH-] = 1x10^-14 |
|
|
What are the 3 laws of thermodynamics (in order)?
|
1) Energy cannot be created or destroyed
2) Entropy increases in spontaneous processes 3) A perfect crystal at 0K has zero entropy |
|
|
What is the change in E equation?
What is the work equation? |
ΔE = q(heat) + w(work)
w = -PΔV |
|
|
1) Temperature wise --> expanding gases ____a____, and compressing gases _____b_____
2) Isobaric? 3) Isochoric? 4) Isothermal? 5) Adiabatic? |
1) a: cool b: heat up
(only apply to non-ideal gases) 2) ΔP = 0 ---> w = 0 3) ΔV = 0 ---> w = 0 4) ΔT = 0 ---> E = 0, E is proportional to T (q = mcΔT) 5) q = 0 |
|
|
1) In this rxn:
2C(s) + O2(g) ---> 2CO (g) is entropy - or +? 2) What two things aren't state functions? 3) What is the ΔH of a bond forming and one breaking? 4) What is the ΔH formula for bond enthalpies? 5) What is the ΔH formula for enthalpies of formation? |
1) Entropy is positive b/c there is a 1 to 2 reactant product ratio (s is not included)
2) q and w (the pathway matters) 3) Forming bond: ΔH < 0 Breaking bond: ΔH >0 4) ΔHrxn = bonds broken(reactants) - bonds formed(products) 5) ΔHrxn = sum of ΔH of products - reactants |
|
|
1) Describe 2 aspects of formation rxns
2) Does the universe prefer exo or endo rxns? why? 3) Using Gibbs eqn, how would you adjust a system that has -ΔH and -ΔS to make it spontaneous? +ΔH and +ΔS? |
1) a: forms 1 mole of product
b: all reactants are elements in their std states 2) Exo b/c the system becomes more stable 3) Lower T for negative and Increase T for positives |
|
|
1) What 3 things fully dissociate?
2) What are the formulas for an acid or base dissociating into water? (used for when ions don't fully dissociate) 3) What are 2 eqns to be used instead of ice tables? 4) Kw = ? 5) pKa + pKb = ? |
1) Strong acids & bases, salts, group 1 & 2's
2) Ka = [H3O+][A-]/[HA] Kb = [OH-][HA]/[A-] 3) [H+] = sqrt(Ka[HA]) [OH-] = sqrt(Kb[A-]) (HA + H2O <---> H+ + A-) 4) [H+][OH-] = KaKb = 1 * 10^-4 @ 25C 5) 14 |
|
|
1) The conjugate bases and acids are considered:
2) Why are salts more soluble in acidic solutions? |
1) Neutral or negligible
2) The H+ can bind to a product and create, example: HF from F-, this decreases the amount of product and causes a shift left |
|
|
1) What is used to calculate the pH of a buffer?
2) What do we want to happen in this eqn - to have max buffering capacity? 3) The buffer range is: 4) How much more acidic is 4 from 7? |
1) pH = pKa + log[A-]/[HA]
2) have the [A-]/[HA] ration = 1, so that pH = pKa 3) +/- 1 from pKa 4) every pH unit is a power of 10, so 4 is 10^3 times more acidic |
|
|
Titrations:
1) Equation? 2) Where is the eq pt for: a: SA/SB b:SA/WB c:WA/SB 3) What does the 1/2 eq pt signify? 4) What does the eq pt signify? |
1. nMV(1) = nMV(2)
2. a: 7 b: <7 c: >7 3. [HA] = [A-] & pH = pKa of acid being titrated/pOH=pKb 4. pH can be determined & molA = molB (the eqn "MaVa = MbVb" can be used) |
|
|
Oxidation States:
1. Elements in their elemental form = 2. Group 1 elements = 3. Group 2 elements = 4. H = ____, unless _____________ (then it = ____) 5. Ag = ____, Zn = ______, Al = _______, Cd = _______ |
1. 0
2. +1 3. +2 4. +1, unless with a metal, then it = -1 5. Al=+3, Zn = +2, Cd = +2, Ag=+1 |
|
|
Electrochemical cells:
1. An anode is always the site of __________ 2. A cathode is always the site of __________ 3. Electrons flow from ________ to __________ 4. Anions flow to the ________, cations to the _________ 5. For non-spontaneous rxns, the anode has a ___ charge and the cathode a ___ charge. For spontaneous rxns? |
1. oxidation (An Ox)
2. reduction (Red Cat) 3. anode to cathode 4. anions to anode, cations to cathode 5. spontaneous=cathode+ and anode-, non-spontaneous is opposite |
|
|
Describe the Galvanic(voltaic) cell and the electrolytic cell
|
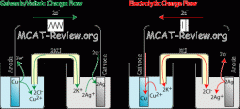
Galvanic is a spontaneous process that produces electricity, while electrolytic is non-spontaneous and consumes electricity
(Red Cat/An Ox) |
|
|
1. Ecell =
2. What is the molar conc. of reactants and products in a std rxn? 3. The nonstandard Ecell eqn = 4. Describe what ΔG, Ecell, and K would equal for a spontaneous rxn |
1. Ecell = Ereduced + Eoxidized
2. reactants & products = both have 1 molar conc. 3. Ecell = E - 0.0592/n * logQ (It's the Nernst eqn --> Ecell = E0cell - (RT/nF)lnQ) 4. ΔG < 0, Ecell > 0, K >Q (if 1 is true, they're all true) |
|
|
Describe electrolysis
What are the 2 quantitative eqns to solve for grams and moles of product? |
Uses a power supply to overcome non-spontaneity and produce elements.
1) Molton (salts) 2) Aqueous (salts dissolved in sol.) Two eqns: (Amps)(Ts)(MW)/(n)(F) = g product (Amps)(Ts)/(n)(F) = moles product look at notes - red notebook |

