![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
251 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Explain the Orbital structure of hydrogen atom, principal quantum number n, number of electrons per orbital.
|
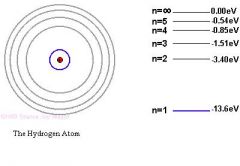
In the Bohr model, the hydrogen electron orbits the nucleus. In quantum mechanics, hydrogen electron exists in a spherical probability cloud around the nucleus. The principle quantum number, n, defines what shell the electron is in. N values start from one: 1,2,3 ...etc. Higher n shells are higher in energy (if subshells are the same). There are n squared orbitals per shell. There are 2 electrons per orbital. Thus, there are 2n^2 electrons per shell. The principle quantum number, n, specifies the electron shell. The angular momentum quantum number, l, specifies the subshell within a certain shell. The magnetic quantum number, m, specifies the orbital within a subshell. Electrons are really small in size and mass compared to the nucleons (protons and neutrons). Protons and neutrons have nearly the same mass, about 1 amu. Since the nucleons are so small compared to the size of the atom, the atom itself is composed mostly of empty space.
|
The energy levels of electrons are quantized like electromagnetic waves and photons. When an electron falls from a higher energy level to a lower energy level, energy is released from the atom in the form of a photon. The photon must have a frequency whihc corresponds to the change in energy of the electron as per ΔE = hf. The reverse is also true: if a photon collides with an electron, it can only bump that electron to antoher energy level and not between energy levels. If the photon doesn't have enough energy to bump the electron to the next level, the electrn will not move from its present level and the photon will be reflected away.
|
|
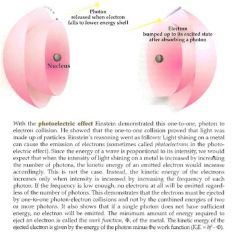
What is the Ground State
and what are excited states? |
Electrons are normally in their ground state (lowest energy level). When they absorb energy (photons), the get promoted to excited states. Excited states are higher in energy than ground states. Excited states come back down to the ground state via release of energy (by emitting a photon and giving off light).
|
|
|
|
What are Absorption
and emission spectra? |
The absorption spectrum shows what frequencies of light are absorbed. The absorption spectrum looks like black lines on a rainbow background. The black lines are the missing absorbed frequencies.The emission spectrum shows what frequencies of light are emitted. The emission spectrum looks like colored lines, created by photons, on a black background. The dark lines in an absorption spectrum appear at exactly the same frequencies as the bright lines in the corresponding emission spectrum. The emission spectrum shifts to a slightly longer wavelength, so wavelengths are not equivalent.
|
|
|
|
What do the quantum numbers l, m, s, mean and what are the number of electrons per orbital?
|
l is the angular momentum quantum number. l are integers that range from 0 to n-1.
spdf: l=0,1,2,3 for s,p,d,f respectively. spdf designates subshells. The subshells each hold a certain number of orbitals. s subshells hold 1 orbital. p holds 3, d holds 5, f holds 7. Each orbital holds 2 electrons. s subshells hold 1x2=2 electrons, p holds 3x2=6, d holds 5x2=10, f holds 7x2=14. A generalized formula for the above pattern: for any subshell, 4l+2 electrons can be held. For a given shell, higher subshells have higher energy. a low shell with a high subshell may be higher in energy than a higher shell with a low subshell. m is the magnetic quantum number: m are integers that range from -l to +l, including zero. The m number tells you the number of orbitals of each subshell. s is the spin quantum number: s is either +1/2 or -1/2. |
|
|
|
What are the common names and geometric shapes for orbitals s, p, d? How can you calculate the total number of orbitals?
|
Orbitals with subshell quantum number l = 0 are called s orbitals. All s orbitals are spherical in shape and have spherical symmetry. In any atom, the size of the s orbital increases as the principal quantum number of the orbital increases but the geometry remains spherical.
Orbitals with subshell quantum number l = 1 are called p orbitals. Since the magnetic quantum number m can be -1, 0, or +1 when the value of the subshell quantum number l is one, p orbitals come in sets of three. In each set, one of the orbitals is aligned along each of the three mutually perpendicular axes of the atom; these axes are traditionally designated x, y, and z. The three 2p orbitals are correspondingly designated 2px, 2py, and 2pz. The p orbitals either as a set or individually do not have spherical symmetry. Orbitals with subshell quantum number l = 2 are called d orbitals; since m can be -2, -1, 0, +1, or +2 when l is two, d orbitals come in sets of five. The d orbitals are usually visualized in three-dimensional representations. An easy way to calculate the number of orbitals found in an energy level is to use the formula n^2. For example, the third energy level (n=3) has a total of 32, or nine orbitals. This makes sense because we know that the third energy level would have 3 sublevels; an s sublevel with one orbital, a p sublevel with 3 orbitals and a d sublevel with 5 orbitals. 1 + 3 + 5 = 9, so the formula n^2 works! An easy way to calculate the total number of electrons that can be held by a given energy level is to use the formula 2n^2. For example, the fourth energy level (n=4) can hold 2(4)2 = 32 electrons. This makes sense because the fourth energy level would have four sublevels, one of each of the named types. The s sublevel hold 2 electrons, the p sublevel holds 6 electrons , the d sublevel holds 10 electrons and the f sublevel holds 14 electrons. 2 + 6 + 10 + 14 = 32, so the formula 2n^2 works! |
|
|
|
How do electrons fill the subshells?
|
Electrons are filled by occupying the lowest energy subshells first. Subshell arranged in increasing energy: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d. Starting from the first row, going across, both hydrogen and helium is 1s. Next row: 2s then 2p. Third row: 3s then 3p. Fourth row: 4s, 3d, then 4p. Fifth row: 5s, 4d, then 5p. Sixth row: 6s, 4f, 5d, then 6p. Last row: 7s, 5f, then 6d. The pattern we get from looking at the periodic table is exactly in the order of increasing energy.
For a given subshell, the columns represent how many electrons are in that subshell. For example, the fifth column of the d subshells contain elements that have 5 electrons in that subshell. The number of columns for each subshell indicate the maximum number of electrons that subshell can hold. For example, the d subshells have 10 columns showing that d orbitals can hold 10 electrons total. |
|
|
|
What is the conventional notation for electronic structure?
|

|
|
|
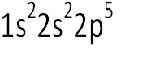
What is the orbital diagram for this orbital?
|
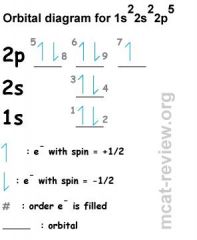
|
|
|
|
What is the Aufbau principle?
|
Shells / subshells of lower energy gets filled first (This is the most obvious rule. For example, 1s fills first, then 2s, then 2p ...etc. Later on, the d subshells get filled after the s.
Aufbau means "building up". B for building up. |
|
|
|
What is Hund's rule?
|
When you fill a SUBSHELL (l subshells) with more than 1 orbital (p, d, f), you first fill each orbital with a single electron and with the same spin (parallel spins). The reason for Hund's rule is that electron-electron repulsion in doubly occupied orbitals make them higher in energy than singly occupied orbitals. Like charges repel each other. As the electrons near each other, the mutual repulsion creates an increase in potential energy. This is why only 2 electrons can fit into one orbital.
|
|
|
|
What is the Pauli exclusion principle?
|
2 electrons in the same orbital must be of different spins.
In its simplest form for electrons in a single atom, it states that no two electrons CAN HAVE THE SAME QUANTUM NUMBERS, that is, if n, l, and ml are the same, ms must be different such that the electrons have opposite spins. So it says above all else, no two electrons can have the same spin, because no two electrons can have the same quantum numbers, including ms. They can have the same n, l, m, but ms must be different. Pauli exclusion - one electron "excludes" the other when it comes to quantum numbers. |
|
|
|
What is special about d4 and d9 elements?
|
Instead of s2d4, it's s1d5 and s1d10 because they want to achieve a half-full or full d subshell.
|
|
|
|
What is the Bohr atom?
|
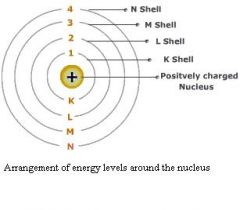
Larger n values (higher energy levels) have larger orbiting radii. An electron orbits the positively charged nucleus in a circular orbit in the same way that the earth orbits the Sun. Electrostatic attraction pulls the electron toward the nucleus. The electron orbits a high speed to prevent it from crashing into the nucleus.
The electron can orbit at different energy levels: n=1, n=2, n=3 ...etc. |
|
|
|
What is the classification of elements into groups by electronic structure?
|
|
|
|

What is effective nuclear charge?
|
Effective nuclear charge = nuclear charge - shielding electrons. Shielding electrons are those that stand between the nucleus and the electron we are interested in. Shielding electrons are those that are in subshells closer to the nucleus (lower in energy) than the electron we are interested in. MCAT questions usually give you a diagram of the Bohr model, in which case, shielding electrons are those that orbits at a smaller radius. The higher the effective nuclear charge for an electron, the more stable it is (higher ionization energy, electrons are not easily knocked off). Effective nuclear charge increases for outer electrons as you go across (left to right) the periodic table.
|
|
|
What are the chemical characteristics of Alkali metals?
|

Single valence electron - low ionization energy, very reactive. Wants to lose that electron to achieve empty valence shell. More reactive as you go down because of increasing radii. Reacts to form ionic oxides like with oxygen to form BaO. Reacts with exothermically water to form metal hydroxides and hydrogen gas. Reacts with acids to form salts and releases hydrogen. Most commonly found in the +1 oxidation state. Alkali metals are soft metallic solids with low densities and low melting points. They are highly reactive, reacting with nonmetals to form ionic compounds. Alkali metals react with hydrogen to form hydrides such as NaH. In nature, alkali metals exist only in compounds.
|
|
|
|
What are the chemical characteristics of alkaline earth metals?
|
2 valence electrons - relatively low in ionization energy, quite reactive. Wants to lose both electrons to achieve empty valence shell. More reactive as you go down because of increasing radii. Reacts with oxygen to form metal oxides. Reacts with water to form hydroxides and releases hydrogen. Reacts with acids to form salts and releases hydrogen. Most commonly found in the +2 oxidation state. Alkaline earth metals are harder, more dense, and melt at higher temperatures than alkali metals. They are less reactive than alkali metals. Heavier alkaline earth metals are more reactive than lighter alkaline earth metals.
|
|
|
|
What are the chemical characteristics of halogens?
|
7 valence electrons (2 from s subshell and 5 from p subshell) - high electron affinity, very reactive.
Wants to gain one electron to achieve full valence shell. More reactive as you go up because of decreasing radii. Reacts with alkali metals and alkaline earth metals to form salts. Most commonly found in the -1 oxidation state. The radioactively stable Group 7A elements (halogens) are fluorine, chlorine, bromine, and iodine. Fluorine and chlorine are diatomic gases at room temperature; bromine a diatomic liquid, iodine a diatomic solid. However, halogens other than fluorine can take on oxidation states as high and POSITIVE as +7 when bonding to HIGHLY ELECTRONEGATIVE atoms like oxygen and fluorine. When in compounds, fluorine ALWAYS has an oxidation state of -1. This means that fluorine makes only one bond, while the other halogens can make more than one bond. |
Hydrogen combines with all the halogens to form gaseous hydrogen halides. The hydrogen halides are soluble in water to form hydrohalic acids. Halogens react with metals to form ionic halids.
|
|
|
What are the physical and chemical characteristics of noble gases?
|
Full valence shell of 8 - high ionization energy couple with low electron affinity. Don't react. Found in the oxidation state of 0. Only the noble gas elements are normally found in nature as isolated atoms. They are all gases at room temperature.
|
|
|

What are the characteristics of transition metals?
|
High conductivity due to free flowing (loosely bound) outer d electrons.
The d-orbitals of a free transition metal atom or ion are degenerate (all have the same energy.) When transition metals form coordination complexes, the d-orbitals of the metal interact with the electron cloud of the ligands in such a manner that the d-orbitals become non-degenerate (not all having the same energy.) Electron transitions between nondegenerate d orbitals gives transition metal complexes vivid colors. Varied oxidation states - but always +. |
|
|

What are the characteristics of representative elements?
|
Representative elements include the s block and the p block of the periodic table.
No free flowing (loosely bound) outer d electrons. Valence shell fills from left (1 electron) to right (8 electrons). Standard nomenclature from left to right: I A, II A, III A, IV A, V A, VI A, VII A, VIII A. Elements are the building blocks of all compounds and cannot be decomposed into simpler substances by chemical means. Any single atom must be one of just over 100 elements. 'A' for elements stands for mass number and NOT atomic number. The atomic number is the identity number of any element. This is not true of the mass number or the number of electrons. Any element may have any number of neutrons or electrons, but only one number of protons. Section A groups are the representative elements or main-group elements and section B groups are the transition metals. |
Two or more atoms of the same element that contain different numbers of neutrons are called isotopes. An atom of a specific isotope is called a nuclide. Isotopes have similar chemical properties. Hydrogen has 3 important isotopes: 1H (protium), 2H (deuterium), and 3H (tritium). Most naturally occuring hydrogen is protium. The 3 isotopes for carbon are 12C, 13C, and 14C. For a given element, the number of neutrons identifies which isotope.
|
|
|
Where are metals, non-metals, and metalloids located?
|
Metals are to the left of metalloids.
Non-metals are to the right of metalloids. Metalloids: all 5 elements contained in a step-like line drawn from the element B to At (mnemonic: BAT). In addition, Ge and Sb are metalloids. The less the electron greediness of the atom (the stronger the effective nuclear charge of the atom), the more the metallic character. That is why the metals are on the left side, because the strength of their nuclear charge is less. To emphasize their loose hold on their electrons and the fluid-like nature of their valence electrons, metals are often described as atoms in a sea of electrons. The easy movement of electrons within metals gives them their metallic character. |
|
|
|
What are the chemical properties of metals?
|
Likes to lose electrons to gain a + oxidation state (good reducing agent). Lower electronegativity - partially positive in a covalent bond with non-metal. Forms basic oxides.
One important periodic trend is metallic character. It tends to increase from left to right and from top to bottom (the bigger the metals, the more metallic they are). |
|
|
|
What are the physical properties of metals?
|
Good conductor of heat and electricity. Malleable, ductile, luster, solid at room temp(except Hg). Metallic character includes ductility (easily stretched), malleability (easily hammered into thin strips), thermal and electrical conductivity and a characteristic luster. Metal atoms easily slide past each other allowing metals to be hammered into thin sheets or drawn into wires. Electrons move easily from one metal atom to the next tranferring energy or charge in the form of heat or electricity. All metals but mercury exist as solids at room temperature.
|
|
|
|
What are the chemical properties of nonmetals?
|
Likes to gain electrons to form a - oxidation state (good oxidizing agent). Higher electronegativity - partially negative in a covalent bond with metal. Forms acidic oxides.
|
|
|
|
What are the physical properties of nonmetals?
|
Poor conductor of heat and electricity. Solid, liquid, or gas at room temp. Brittle if solid and without luster. Nonmetals have diverse appearances and chemical behaviors. Generally, they have lower melting points than metals. Nonmetals form covalent oxides like SiO2 or CO2. Hydrogen is unique and its chemical and physical characteristics do not conform well to any family. It is a nonmetal. Under most conditions, it is a colorless, odorless, diatomic gas.
|
|
|
|
What is the oxygen group and what is chemically similar to oxygen?
|
The group (column) that contains oxygen.
Oxygen and sulfur chemically similar. Group is also called family in the periodic table and they are the columns. The rows in the periodic table are called periods. Elements in the same family on the periodic table tend to have similar chemical properties. For example, they tend to make the same number of bonds and exist as similarly charged ions. |
|
|
|
What is the electronic structure of the representative elements?
|
Representative elements include the s block and the p block of the periodic table.
No free flowing (loosely bound) outer d electrons. Valence shell fills from left (1 electron) to right (8 electrons). |
|
|
|
What is the electronic structure of the noble gases?
|
Full valence shell of 8 - high ionization energy couple with low electron affinity.
|
|
|
|
What is the electronic structure of transition metals?
|
High conductivity due to free flowing (loosely bound) outer d electrons.
In the presence of ligands (when in a chemical complex), the d orbitals become nondegenerate (different in energy). Electron transitions between nondegenerate d orbitals gives transition metal complexes vivid colors. Varied oxidation states - but always +. |
|
|
|
What are valence electrons?
|
Electrons in the outer shell.
Ranges from 1 to 8 from left to right of the representative elements. The valence electron rule does not apply to transition metals. Charge density is the relationship of the charge around an atom and how close that charge is to the nucleus (how small the atom is). So if you have 2 atoms with the same ionic charge, but 1 atom has a smaller atomic radius than the other, the atom with the smaller atomic radius has a higher charge density. If you have 2 atoms with different ionic charges, like Al3+ and Mg2+, Mg2+ has a lower ionic charge and it has a larger atomic radius, so it has a lower charge density than Al3+. Charge density could be the density of positive charges and the density of negative charges - it all depends on how large or small the radius of the atom is. If it has a larger radius, then the charges are more spread out, and the charge density is lower. |
|
|
|
What is the definition of first and second ionization energies?
|
Definition of first ionization energy: The energy needed to knock off the first valence electron.
Definition of second ionization energy: The energy needed to knock off the second valence electron. The term ionization energy of an atom or molecule is the MINIMAL energy required to remove an electron from the atom. It increases from left to right and from down to up on the periodic table. When a photon or radiation, like x-rays and gamma rays, ionizes another atom, it knocks off an electron. The kinetic energy of the ejected electron is the difference between the eV of the photon or radiation and the eV of the atom that it ionized. The ionization energy is the same as the energy of the photon, because the photon (the light, the radiation, the x-ray, the gamma ray), comes in and pulls the electron out of the orbit. So you can equate ionization energy E = hf. Once you find frequency, you can find wavelength by using the light's wave speed, which is 3.0 x 10^8 m/s. The electron volt is a unit of energy equal to approximately 1.602×10−19 J. By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt. Thus it is 1 volt (1 joule per coulomb) multiplied by the electron charge (1 e, or 1.6 ×10^ −19 C). Therefore, one electron volt is equal to 1.6 ×10−19 J. A particle with charge q has an energy E=qV after passing through the potential V; if q is quoted in integer units of the charge and the terminal potential in volts, one gets an energy in eV. In chemistry, it is often useful to have the molar equivalent, that is the kinetic energy that would be gained by one mole of electrons (6.022×10^23 passing through a potential difference of one volt. This is equal to 96.5 kJ/mol. Atomic properties like the ionization energy are often quoted in electron volts. |
By definition, the atom being ionized is gaseous.
|
|
|
How do you predict the ionization energy from electronic structure for elements in different groups or rows?
|
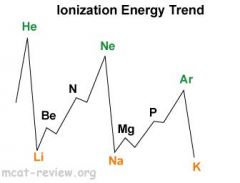
Ionization energy decreases as you go down because of increasing radii.
Ionization energy increases as you go right because of decreasing radii. Highest peaks are noble gases. Lowest troughs are alkali metals. Second ionization energy is always higher than the first ionization energy (usually a lot higher). Ionization is when the potential energy between the electron and the nucleus of an atom has reached zero - since the electrostatic potential between them is U = kq1q2/r, when ionization occurs, the distance for r has reached infinity (the electron is completely pulled away), and the negative value has gotten so small that it has reached 0 - basically, the potential energy has increased to zero. |
|
|
|
What are the first and second ionization energies like for alkali and alkaline metals?
|
Alkali metals and hydrogen: first ionization energy very low. Second ionization much higher.
Alkaline earth metals: first ionization energy low. Second ionization energy also low, but higher than the first for alkaline earth metals. The second ionization energy is always much greater than the first because when one electron is removed, the effective nuclear charge on the other electrons increases. Ionization energy generally increases along the periodic table from left to right and from bottom to top. This trend is explained by the effective nuclear charge. The effective nuclear charge increases when moving across the period to the right making it tougher to knock off an electron. Although the effective nuclear charge also increases when moving down the periodic table (because of the higher atomic number), the DISTANCE of the electron from the nucleus increases as well, thus decreasing the electric field and the force at the point of the electron (E = F/d). The decreased electric field and force have less strength to hold the electron to the atom. |
|
|
|
What are the "islands of stability" in the periodic table and how do they affect the ionization energy? How would you rank IE for
C, N and O? |
The answer is C<O<N NOT C<N<O. C is lower than N because N has higher effective nuclear charge.
N is higher than O because p3 doesn’t want to lose electron O is lower than N because p4 likes to become p3 offsetting effective nuclear charge |
|
|
|
What are the electron configurations for Cr, Mo, W and ions like Fe2+, Ru2+, Ir3+?
|
They all provide an electron from s2 to create a s1d5 electronic configuration.
|
|
|
|
What are the electron configurations for Cu, Ag, Au and ions like Tl2+, Au-?
|
They all provide an electron from s2 to create a s1d10 electronic configuration.
|
|
|
|
What are the electron configurations like for the large metals with valence p1 to p4 electrons.?
|
Remove the electrons so that a filled d10 can be created as soon as possible. Electrons lost from large ions come off in the following order: p first, s second, d last. In+ loses its electron from p orbit, e- configuration to make a d10s2
In3+ loses its electrons from p first and then the s orbits to make a d10 |
|
|
|
What is the ranking for the half-filled and filled shells in the periodic table?
|
p6 >>>>>>>> d10 >>>> d5 > p3 >> s2
Relative extent of stability from filled and half filled shells. |
|
|
|
What is electron affinity and what is the electron affinity trend?
|
Electron affinity is the amount of energy released when something gains an electron (how easily it can gain an electron).
The ionization energy is a specific amount of energy involved in removing an outer shell electron (the highest energy electron because it's valence) from a neutral gaseous atom. This is the first ionization energy. Electron affinity is the energy gained or lost when an electron is added to a neutral atom. It's another specific kind of situation. The electronegativity is the relative strength that an atom has for the electrons it shares with another atom in a chemical bond. Electronegativity is like the strength the atom has in a tug-of-war with another atom over the electrons it shares in bonding. When you go across a row, electron affinity increases, which means that the atoms will accept electrons more readily and the electron affinity becomes more negative (less positive). Electron affinities have negative values. For example, the first electron affinity of chlorine is -349 kJ mol-1. By convention, the negative sign shows a RELEASE of energy. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. If energy was gained (as in ionization energy), then the sign would be positive. This is more easily seen in symbol terms. X2(g) + e- yields X-(g) . It is the energy released (per mole of X) when this change happens. Ionisation energies are always concerned with the formation of positive ions (because an electron is removed). That is why electron affinity becomes more negative when atoms gain these electrons. |
Electron affinity ist he willingness of an atom to accept an additional electron. It is related to effective nuclear charge. Electron affinity is more exothermic to the right and up on the periodic table. The noble gases do not follow this trend. Electron affinity values for the noble gases are endothermic.
|
|
|
What are the trends of electron affinity?
|
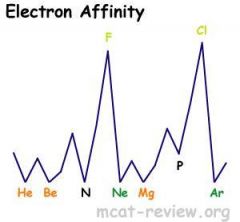
As you go down a group, electron affinity decreases because of larger radii.
As you go across (left to right) a row, electron affinity increases. Highest peaks are for the halogens. Lowest for noble gases. |
|
|
|
How do filled subshells and half-filled p subshells affect electron affinity?
|

Consider what is going on with electron configuration around p2 p3 and p4. The ranking for
EA for C, N and O is N<C<O NOT C<N<O. C has a more positive EA because adding e- makes p3 N has a more negative EA because adding e- makes p4 O has the most positive EA due to higher effective nuclear charge |
|
|

What is electronegativity?
|
Electronegativity is a chemical property that describes the ability of an atom to ATTRACT electrons (or electron density) towards itself in a BOND. An atom's electronegativity is affected by both its atomic weight and the distance that its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. Fluorine is the most electronegative atom. Electronegativity increases toward the top right. The Electron affinity of a molecule or atom is the energy change when an electron is added to the neutral species to form a negative ion. The term ionization energy (EI) of an atom or molecule is the minimal energy required to remove an electron from the atom or molecule. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. No electronegativity difference between two atoms leads to a pure non-polar covalent bond. A small electronegativity difference leads to a polar covalent bond. A large electronegativity difference leads to an ionic bond. Electronegativity increases across a period because the number of charges on the nucleus increases. That attracts the bonding pair of electrons more strongly. As you go down a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Electronegativity is used in the context of bonding - electronegativity is the relative strength of attraction that an atom has for the electrons that is shares with another atom in a chemical bond. Oxygen is SUPER electron greedy - it is only second in electronegativity to Fluorine.
|
Electronegativity is related to the effective nuclear charge. Electronegativity values are undefined for noble gases.
|
|
|
What are the electronegative characteristics for some representative elements and groups?
|
Fluorine is the most electronegative element.
Things around fluorine are highly electronegative: N, O, F, Cl, Br. Halogens are electronegative, especially toward the top of the group. Noble gases can be very electronegative if they participate in bond formation (Kr and Xe). Non-metals are more electronegative than metals. Covalent bond is a sharing of electrons between elements. The more electronegative element in a covalent bond gets a larger share of the electrons and has a partial negative charge The less electronegative (more electropositive) element in a covalent bond gets a smaller share of the electrons and has a partial positive charge. If the electronegativity difference is too great, an ionic bond occurs instead of a covalent one. Ionic bonds result from a complete transfer of electrons from the electropositive element to the electronegative element (resulting in oppositely charge species that attract each other via electrostatic interaction). |
|
|
|
Explain how electron shells and effective nuclear charge change as you go across and down the periodic table.
|
Electron shells are defined by the principle quantum number - the n value.
Going down the periodic table means jumping to the next shell. As you fill to the next shell (Ne to Na), the effective nuclear charge decreases because the old shell stands in between the nucleus and the new shell. Filling to the next shell causes a jump in atom size because of decreased effective nuclear charge. As you go down a group (Na to K), the atomic size increases even though the effective nuclear charge stay the same, because higher shells have a larger radius than lower shells. Going across the periodic table means filling up the same shell (by going through subshells). As you fill up a shell, the effective nuclear charge increases because the atomic number (protons) is increasing while the same-shell electrons you add do not shield one another. With increasing effective nuclear charge, the electrostatic attraction (F=kQq/r^2) between the nucleus and the electrons increases, so the atom become more compact. The increasing effective nuclear charge and electrostatic attraction is why going across a periodic table means decreasing atomic size. |
|
|
Explain the trend of atomic radii.
|
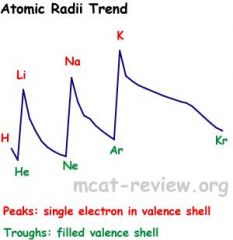
Size increases as you go down a column.
Size decreases as you go across (to the right of) a row. Atomic sizes may overlap if you zigzag on the periodic table. An Angstrom is 1/10th of a nanometer. 1 angstrom = 1.0 × 10^-10 meters. Small atoms have less room to stabilize charge by spreading it out. This makes them bond more strongly to water resulting in greater heats of hydration. Because Be in its ionic form is not large enough to stabilize its charge, it forms a covalent oxide, whereas other alkaline earth metals make ionic oxides. This means that BeO is amphoteric whereas other alkaline earth metal oxides are basic. The second period elements are small enough to form strong pi bonds while their larger third row family members only form weak pi bonds, if they form pi bonds at all. Atoms without d orbitals cannot form more than 4 bonds. The p orbitals on atoms that are too big don't significantly overlap, so the large atoms can't easily form pi bonds. |
|
|
|
What is the equation for electrostatic energy?
|
Electrostatic Energy = Electrostatic potential x charge = kq1/r x q2 = kq1q2/r
Electrostatic energy is negative because q1 and q2 are opposite in charge (If q1 and q2 are not opposite in charge, then they would repel each other, and no ionic bond would form). Frequently, the negative sign is dropped and only the magnitude of the electrostatic energy is used. The greater the magnitude of electrostatic potential, the stronger the ionic bond. Strong ionic bonds are promoted by high charge magnitudes (q values) that are close together (small r value). Ions that form strong ionic bonds have high charge density, that is, the charge to size ratio is high. |
|
|
|
What is lattice energy?
|
Lattice energy measures the ionic bond strength.
Lattice energy is the energy required to break the ionic bond. The larger magnitude of the lattice energy, the stronger the ionic bond and the harder it is to break. The lattice energy is proportional to the electrostatic attraction between the ions. The lattice energy is the sum of the electrostatic potential energy, calculated by summing interactions between cations and anions. |
|
|
|
What is electrostatic force?
|
Coulomb's law: F = kq1q2/r2
Larger charge magnitudes + charges being closer together → greater electrostatic force. The Coulomb's constant, k, is 9 x 10^9. Opposite charges attract (negative F), same charges repel (positive F). If q1 doubles, the electrostatic force doubles. If r halves, the electrostatic force increase by a factor of 22 = 4. Coulomb's law is analogous to the universal law of gravitation: F = Gm1m2/r2 G is analogous to k and m is analogous to q. The big difference is that G is tiny compared to k, because gravitational force is weaker compared to the much stronger electrostatic force. |
|
|
|
What are covalent, pi, and sigma bonds?
|
The covalent bond results when there is a sharing of electrons between two atoms, resulting in the overlap of their electron orbitals.
σ bonds are single bonds. They also make up the first bond of double and triple bonds. π bonds are double and triple bonds. They make up the second bond in a double bond, and both the second and the third bond in a triple bond. There are 2 types of forces acting on a bond - there is the attractive force between the nucleus of the atom and the electrons between the atom. The closer these two charges are together, the lower the potential energy (the more stable the atom is because the energy in is lowered). So if you try to pull the bond apart, you have to do work because this increases the potential energy (U = kq1q2/r and r increases). However, if you push the atoms together too much, the repulsive forces between the positively charged nuclei take over, and there is an increase in the potential energy from them being too close. So there is a intermolecular distance for each bond that keeps it at a stable position. |
The bond length is the point where the energy level ist he lowest. Two atoms will only form a bond if they can lower their overall energy level by doing so. Nature tends to seek the lowest energy state.
|
|
|
How are hybrid orbitals produced and what are the respective geometries of sp3, sp2, and sp orbitals?
|
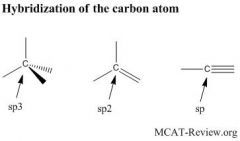
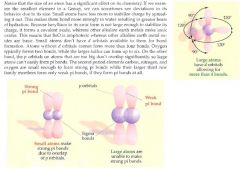
Hybrid orbitals are produced by hybridizing (mixing) electron orbitals to produce geometries that facilitate bonding.
Sp3: a hybrid between one s with 3 p orbitals. Tetrahedral, pyramidal, or bent in geometry. Contains single bonds only. 109.5° Sp2: a hybrid between one s with 2 p orbitals. Trigonal planar in geometry. Contains a double bond. 120° Sp: a hybrid between one s with one p orbital. Linear in geometry. Contains a triple bond. 180°. dsp3: 90°, 120°. Shape: trigonal-bypyramidal, seesaw, T-shaped, linear d2sp3: 90°. Shape: octahedral, square pyramidal, square planar Hybrid orbitals are most commonly used with carbon as the center atom. If you are only looking at single (sigma) bonds, the number of these new hybrid orbitals must be equal to the numbers of atoms and non-bonded electron pairs surrounding the central atom! So ammonia (NH3) is sp3, water is sp3, methane (CH4) is sp3 because they have 4 groups (bonded and electron pairs) around it. BF3 is sp2 because it only has 3 groups around it. BeCl2 is sp because it only has 2 groups around it. Groups with double and triple bonds around it only count the sigma bond of the double and triple bonds in the hybridization. Ethene (C2H4) is sp2 because it is double bonded (1 sigma bond) and has 2 other groups around it (2 hydrogens). Acetylene (C2H2) is sp hybridized because it has the 1 sigma bond of its triple bond and 1 other group around it (the hydrogen). |
The more s character a bond has, the more stable, the stronger, and the shorter it becomes. This is because an sp orbital has 50% s character and 50% p character, an sp2 orbital has 33.3% s character and 66.7%p character, and an sp3 orbital has 25% s character and 75% p character. Lone pairs, pi electrons, and ring strain can distort the predicted bond angles. Lone paris and pi electrons require more room than bonding pairs. For example, the lone pairs on water make the bond angle 104.5° instead of the expected 109.5°. A pi bond has 100% p character and a sigma bond has 100% s character. A pi bond is at a higher energy level than a sigma bond.
|
|
|
What is the valence shell electron-pair repulsion (VSEPR) theory? What are the predictions of shapes of molecules
(e.g., NH3, H2O, CO2)? |
The VSEPR theory is used to predict the geometry of molecules.
The shapes of molecules are determined by the molecular geometry. Radicals also count as an electron pair. The VSEPR number is the total number of bonds + unbonded electron pairs. When calculating the VSEPR number, always use the electron/bond configuration about the central atom. NH3 has a vsepr number of 4 (3 bonds to H and 1 unbonded pair). If you look up the table for VSEPR # = 4 and # unbonded electron pairs = 1, then you'll find that NH3 is trigonal pyramidal. It has an angle of a little less than tetrahedral because the lone pair pushes the hydrogen bonds away - it repulses the electrons within the hydrogen bonds a little - so the angle of ammonia is about 107°. H2O has 2 bonds, 2 unbonded electron pairs - it is bent. H2O has 2 lone pairs on the oxgen, so those lone pairs are more repulsive than the 1 lone pair on ammonia, so it pushes the 2 hydrogen bonds a little more strongly. The H2O bond is like a tetrahedral bond, but the angle is even more smaller than the angle for ammonia - it's about 105°. Both the H2O and the NH3 are based on the tetrahedral shape because they both have the same electron density (4 regions of electron density), but because the H2O and the NH3 have lone pairs, the lone pairs distorts the ideal tetrahedral angle and pushes the bonded pairs closer together. CO2 has 2 double bonds and 0 unbonded electron pairs - it is linear. |
|
|
|
What are the shapes for VSEPR #2-6?
|
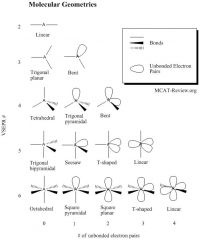
|
|
|

What are Lewis Dot Structures?
|
Every dot represents 1 electron. Every line represents 1 bond (2 electrons). A "lone pair" is represented by two dots.
Formulas are drawn in such a way that an octet is achieved on each atom. Exceptions include the boron column (they form 3 bonds and have a six-tet), large elements (3rd row and below such as the 10-tet P in PO43- and the 12-tet S in SO42-), and radicals (compounds with an odd # total electrons that result in a single, unpaired electron). All electrons in a bond is shared and can be used to satisfy the octet for both atoms on either side of the bond. Lewis structures for elements in the same column (group) of the periodic table are similar to one another. For example, sulfur can be substituted for oxygen in lewis structures of oxygen. |
|
|
|
What kinds of bonds form with carbon, oxygen, and nitrogen?
|
Carbon: 4 bonds total (meaning 4 total bonds. It can either be 4 single bonds or two double bonds ...etc) and no lone pairs. eg. CH4, CO2
Oxygen: O can be O: 2 bonds total, 2 lone pairs. eg. H2O, O2 O1-: 1 bond, 3 lone pairs, formal charge of -1. O1+: 3 bonds, 1 lone pair, formal charge of +1. Nitrogen: N can be N: 3 bonds total, 1 lone pair. eg. Amine or ammonia NH3 N+: 4 bonds, 0 lone pair, formal charge of +1. eg. Ammonium NH4+ All the 4A elements can form 4 covalent bonds with nonmetals. All but carbon can form 2 additional bonds with Lewis bases. Of the 4A elements, only CARBON forms STRONG pi bonds to make STRONG double and even triple bonds. Group 5A elements can form 3 covalent bonds. In addition, all 5A elements EXCEPT NITROGEN can form 5 covalent bonds by using their D ORBITALS. These elements can FURTHER bond with a Lewis base to form a SIXTH covalent bond. Nitrogen forms STRONG pi bonds to make double and triple bonds. The other 5A elements cannot make pi bonds. Nitrogen can also form 4 covalent bonds by donating its lone pair of electrons to form a bond. |
Group 6A elements are called the chalcogens. Oxygen and sulfure are the important chalcogens for the MCAT. Oxygen is the second most electronegative element, second to only fluorine. Oxygen is divalent and can form STRONG pi bonds to make double bonds. In nature, oxygen exists as O2 (dioxygen) and O3 (ozone). Oxygen typically reacts with metals to form metal oxides. Alkali metals form peroxides (Na2O2) and super oxides (KO2) with oxygen. The most common form of pure sulfur found in nature is the yellow solid S8. Metal sulfides, such as Na2S are the most common of sulfur found in nature. Sulfur can form 2, 3, 4, or even 6 bonds.
|
|
|
What kinds of bonds form with halogens, hydrogens, carbocations, carbanions, and borons.
|
Halogens: 1 bond, 3 lone pairs. eg. CCl4
Hydrogen: 1 bond, 0 lone pair (exception to octet rule). Carbocation: C+ has 3 bonds, no lone pairs, formal charge +1. Carbanion: C- has 3 bonds, 1 lone pair, formal charge -1. Boron: 3 bonds, 0 lone pairs (exception to the octet rule). eg. BH3 |
|
|
|
What is the lewis dot structure of hydrogen and boron?
|
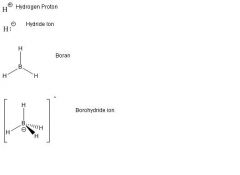
|
|
|
|
What are the carbon lewis structures?
|
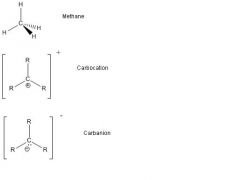
|
|
|
|
What are the nitrogen lewis structures?
|
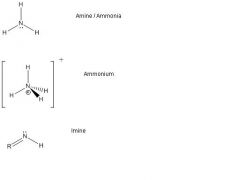
|
|
|
|
What are the oxygen and halogen lewis structures?
|
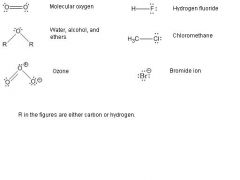
|
|
|
|
What are resonance structures?
|
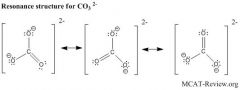
When there are more than 1 satisfactory Lewis structures for a molecule, they are called resonance structures.
The structure of the molecule "shifts" between each of its resonance structure. The molecule spends most of its time in the most stable resonance structure. Stable properties: Octet rule is satisfied in every atom (except for boron group and hydrogen). No formal charges. If there must be formal charges, like charges are apart and unlike charges are close together. |
|
|
|
What are fomal charges?
|
Formal charge = valence electron # in the unbonded atom - electron # in the bonded atom.
Electron # in the bonded atom = dots around the atom + lines connected to the atom. The dots around the atom represent electrons that are held entirely by the atom. The lines connected to the atom represent bonding electron pairs, in which the atom only gets one of the two electrons. Formal charges (other than 0) must be labeled next to the atom with the formal charge. |
|
|

What are common formal charges of oxygen, carbon, nitrogen, halogen, and boron?
|

Oxygen with only a single bond: -1.
Oxygen with no bond but have an octet: -2. (Oxygen usually exists as the diatomic O2 and have a double bond to themselves) Carbon with only 3 bonds: either +1 if carbocation or -1 if carbanion. Nitrogen with 4 bonds: +1. Halogen with no bonds, but have an octet: -1. (Halogens usually exist as a diatomic and have a single bond to themselves such as Cl2) Boron with 4 bonds: -1. eg. BH4- |
The elements that tend to exist as diatomic molecules are hydrogen, oxygen, nitrogen, and the halogens. When these elements are discussed, it is assumed that they are in their diatomic form unless otherwise stated. In other words, the statement, "Nitrogen is nonreactive" refers to N2 and not N.
|
|
|
What are lewis acids and bases? What are Arrhenius acids and bases?
|
Lewis acid accept electron pairs. They don't have lone pairs on the central atom. eg. BF3
Lewis bases donate electron pairs. They have lone pairs on their central atom. eg. NH3 An Arrhenius acid is anything that produces hydrogen IONS in aqueous solution, and an Arrhenius base is anything that produces hydroxide IONS in aqueous solution. This definition only covers AQUEOUS solutions. The Lewis definition is the most general and includes all the acids and bases in the Bronsted-Lowry and more. Lewis acids include molecules that have an incomplete octet of electrons around the central atom, like AlCL3 and BF3. They also include all simple cations except the alkali and the heavier alkaline earth metal cations. The smaller the cation and the higher the charge, the stronger the acid strength. Fe3+ is a common example of a Lewis acid. Molecules that are acidic only in the Lewis sense (accept electrons) are not generally acids unless they are referred to explicitly as Lewis acids. |
Aqueous solutions always contain both H+ and OH-. An aqueous solution containing a greater concentration of H+ than OH- is acidic, while an aqueous solution containing a greater concentration of OH- than H+ is basic. An aqueous solution with equal amounts of H+ and OH- is neutral.
|
|
|
What is partial ionic character?
|
Covalent bonds between atoms with dissimilar electronegativities have a partial ionic character.
Hydrogen chloride is composed of diatomic molecules, each consisting of a hydrogen atom H and a chlorine atom Cl connected by a covalent single bond. Since the chlorine atom is much more electronegative than the hydrogen atom, the covalent bond between the two atoms is quite polar. HCl is not ionic, but NaCl is ionic. HCl is not ionic because that has been determined by an electronegativity table - the difference in electronegativity is not great enough to be ionic. The large electronegativity difference between oxygen hydrogen causes the molecules to be very polar. This contributes to the strength of hydrogen bonding in water; these strong intermolecular attractions cause the temperature at which water boils to be high. |
|
|
|
What is the role of electronegativity in determining charge distribution?
|
The more electronegative atom receives a partial negative charge.
The less electronegative atom receives a partial positive charge. When strong electronegative atoms pull the electrons toward it, it is actually decreasing the electrostatic potential energy (unlike charges of the protons and the electrons). So by it decreasing the electrostatic potential energy, it is pulling that bond inside of a deeper well, and it will make it more difficult to pull them apart (to break the bond). These bonds are lower in energy than the unbounded atoms and electrons. This also creates an exothermic reaction because energy is released, since the products are lower than energy than the reactants. These bonds also cause a decrease in internal energy change because of the lower potential energy. These bonds are also stronger because you have to pull these electrons away - it takes more work to pull these electrons away from each other. Electronegativity is at the heart of oxidation-reduction - because those more electronegative atoms like to get reduced and those less electronegative atoms like to get oxidized. |
Intermolecular and intramolecular forces are electrostatic. Electrostatic interactions is the reason for dipoles.
|
|
|
What are dipole moments?
|
Molecules with asymmetrical partial charge distribution have a dipole moment. eg. H2O has a dipole moment because the molecule is bent and the oxygen-side of the molecule is partially negative.
Dipole moment depends on charge and distance. The greater electronegativity difference, the greater the charge and hence the dipole moment. The greater the distance separating the charges, the greater the dipole moment. Molecules with symmetrical partial charge distribution do not have dipole moments. eg. CCl4 do not have a dipole moment because the partially negative chlorine atoms are arranged symmetrically in a tetrahedron. The symmetry cancels out their individual dipole moments. Things with a dipole moment are said to be polar. Are the individual bonds in CCl4 polar? Ans: yes. Is the entire molecule CCl4 polar? Ans: no. Carbon dioxide is non-polar. Silver chloride is polar because silver chloride is ionic. |
|
|
|
What is Absolute temperature, K?
|

K = °C + 273
F = °C x 1.8 + 32 |
|
|
|
What is pressure?
|
Pressure is the force exerted over an area: P = F/A
1 Pascal is a N/m. 1 atm = 1x10^5 Pa. Due to gravity, the atmosphere exerts a pressure of 101 kPa at sea level. For convenience, 101 kPa = 1 atm. Pressure decreases at higher elevations. 1 atm = 101 kPa = 101,000 Pa = 760 mm Hg = 760 Torr. When performing P = F/A calculations, make sure that F is in Newtons, A is in meter squared and the resulting P will be in Pascals. You can then convert the Pascals to whatever units the answer choices are in. This pressure also coincides with pressure of a gas. If a force causes a gas to be compressed, the gas now has a certain pressure from the force from the equation F = P/A. If the force is constant, then the pressure is constant. |
You can think of the atmosphere as a sea of air. As you move closer to the top of this sea, its depth (y) decreases. Near the top, you have fewer molecules above you, which means less weight and lower pressure.
|
|
|
What is the simple mercury barometer?
|
The mercury barometer measures atmospheric pressure by allowing the atmospheric pressure to "push" on a column of mercury.
The barometer is open at one end and closed off (vacuum) at the other. The atmosphere "pushes" at the open end, which results in the mercury rising up in the closed end. The measured atmospheric pressure P = F/A. F is the weight of the mercury that got pushed up and A is the cross-section area of the column that the mercury got pushed through. Standard mercury barometers are calibrated such that 1 atm of pressure will push the mercury up by 760 mm. For convenience, mm Hg is also called the Torr. So, you don't have to do the P=F/A calculation to find out the pressure reading from a barometer. Just know that 1 atm = 760 mm Hg = 760 torr. |
|
|

Memorize: The molar volume of an ideal gas at 1 atm is 22.4 L/mol at 0° C
|

You must memorize this: ideal gases occupy 22.4 L per mol of molecules.
Do not get this mixed up - it is 22.4 liters per mole, not the other way around. The way to remember this is that the mol is a huge number - 6.02E23 molecules. These gazillions of molecules occupy a lot of space - 22.4 L to be exact. It's also at 0° C and 1 atm. This is what would need to be used to convert gases from g/L to g/mol. However, if you have 2 gases in a closed container, the volume of the gases will equal the volume of the container - because that's what ideal gases do. |
They don't have their own molecular volume, their volume is the volume of the container. The average kinetic energy of the molecules does not change with time. The molecules bounce and bounce but, on average, do not slow down as long as the temperature of the gas remains constant. Energy can be transferred between molecules during collisions but not lost because the collisions are perfectly elastic (not sticky)
The average kinetic energy of the molecules is proportional to absolute temperature (A result of Thermodynamics). At a given temperature the molecules of all species of gas, no matter what size shape or weight, have the same average kinetic energy and average speed. However, the individual gas molecules have different speeds because the speed depends on the mass (1/2mv^2 = 3/2nRT). Kinetic energy for gases is KE = 3/2kT, where k represents R/(Avogadro's number, the number of molecules of gases). So if you have 2 gases with the same kinetic energy, the average kinetic energy of the MOLECULES of those two gases will be the same, even if their moles are different. If their number of MOLES are different, then the root mean square speed (average speed) of the gas molecules will be different (1/2mv^2). |
|

What is the definition of an ideal gas?
|

An ideal gas consists of pointy dots moving about randomly and colliding with one another and with the container wall. The ideal gas obeys the kinetic molecular theory of gases and has the following properties.
Random molecular motion. No intermolecular forces. No (negligible) molecular volume. Perfectly elastic collisions (conservation of total kinetic energy). You can treat gases as ideal gases at: Low pressures High temperatures Ideal gases behave according to the ideal gas law. Gases are composed of molecules whose size is negligible compared to the average distance between them, so size is not considered for ideal gases (or for Graham's law). For an ideal gas, there is no other kind of internal energy except for kinetic energy: KE = 3/2nRT. There's no potential energy from electrostatics or gravitation. If the temperature is going up, then the internal energy is going up for an ideal gas. If the temperature is constant, the internal energy is constant. So the molar heat capacity of an ideal gas is 3/2R, because this is the amount of energy that it takes to change 1 mole of gas by 1 K. The average molecular kinetic energy is proportional to the absolute temperature of a gas. If two gases in a container are at the same temperature, then they have the same average kinetic energy. If the internal energy of a gas is the same, the temperature change is the same. |
KE = 3/2nRT is the average translational kinetic energy found from the root-mean-square (rms) velocity. rms velocity is the square root of the average of the squares of the molecular velocities. rms velocity is slightly greater than average speed. KE = 3/2nRT is valid for any fluid system, including liquids. Notice that the kinetic energy is is the average kinetic energy for a MOLE of gas molecules and not the energy of every, or even maybe any, of the MOLECULES themselves. A single gas MOLECULE chosen at random may have almost any kinetic energy associated with it.
|
|
When do deviations from the ideal gas behavior occur?
|

Deviation from the ideal occurs at high pressure and low temperature. At these conditions, the gas molecules are "squished" together. When the gas molecules are so close together, they experience intermolecular interactions. Also, the molecular volume becomes significant when the total volume is squished down so much. The intermolecular attractions will cause collisions to be sticky and inelastic. At the extremely high pressures and low temperatures, gases cease to be gases at all - they condense into liquids. Intermolecular attractions could be attractions like hydrogen bonding. Deviations tend to occur with increased molecular mass and complexity - increased molecular mass and complexity tend to coincide with greater molecular volume and greater intermolecular forces. If you are looking at a chart that shows deviations of PV/RT = 1, and you know that a gas has a certain temperature and pressure, then you will be concerned with the devations of volume. If PV/RT < 1 and the pressure is low, then that means that the ideal gas is supposed to have a PV/RT = 1, so it is higher than the calculated amount in the graph, so volume must be greater for the ideal gas (and volume is smaller for the real gas). If PV/RT >1, then that means that at that given pressure, the ideal gas is supposed to be PV/RT = 1 and the calculated amount is too high - so the volume must be too high for the real gas and the ideal gas is supposed to have a lower volume. When pressure is low, the volume is supposed to be high ideally, but if it's not high enough, PV/RT < 1, when pressure is high, the volume is supposed to be lower ideally, but if it's not low enough, then PV/RT >1. A greater than 1 value for PV/RT indicates a deviation due mainly to volume because real volume is greater than ideal volume (ideal volume is negligible). The deviation could also be due to intermolecular forces, but if pressure is sufficiently high, volume dominates.
|
A typical real gas is a loose collection of weakly attracted atoms or molecules moving rapidly in random directions. In a gas, the volume of the molecules accounts for about 0.1% of the total volume occupied by the gas. By comparison, molecules in a liquid account for about 70% of the total volume occupied by the liquid.
|
|
What is the ideal gas law?
|
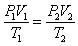
Ideal gas law PV=nRT, where P is pressure, V is volume, n is # mols of gas, R is the gas constant, and T is temperature.
Combined gas law: Because nR is constant (n is the # mols and R is the gas constant), PV/T must also be constant. Boyle's law and Charles' law can all be derived from the combined gas law. It is a good approximation to the behavior of many gases and so if you have 2 containers, and one contains 1L of nitrogen gas and one contains 1L of oxygen gas, and they are both at STP, if they behave ideally, they will have the same number of molecules and kinetic energy because one mole of an ideal gas will occupy a volume of 22.4 liters at STP. So since they have the same number of moles, and they are at the same STP (same temperature), they have the same KE = 3/2nRT. Avogadro's number of molecules 6.022x10^23 is equal to one mole. If you have a reaction between two ideal gases, if their P, V and T are the same before and after the reaction, then their number of moles would be the same too. So, the chemical equation would have to be a balanced equation that has an equal number of moles of the reactants and products. If you have 2 gases, but 1 gas can only use its kinetic energy to move linearly, while another gas can use its kinetic energy to move linearly and rotate and vibrate, the vibrate that can do more than linear motion will have a higher heat capacity because it can use up more energy before the energy gets converted into heat. |
Since temperature dictates the average kinetic energy of molecules in a gas, the gas molecules of each gas in any gaseous mixture must have the same average kinetic energy. Gases can contribute to a pressure that matches the stoichiometric coefficient of the gas in the balanced equation.
|
|
|
What is Boyle's law?
|
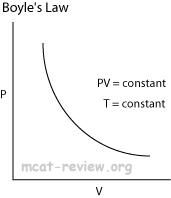
Boyle's law: at constant temperature, P1V1 = P2V2
These are for isothermal thermodynamic systems (constant temperature). |
|
|
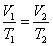
What is Charles's Law at constant pressure?
|
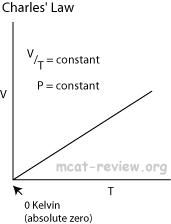
Charles's law extrapolates to absolute zero, where volume also goes to zero (this is only an extrapolation). Absolute zero is the temperature at which the pressure of a gas becomes zero when a plot of pressure versus temperature for a gas is extrapolated. The pressure of a gas approaches zero when the temperature is about -270°C. When more accurate measurements are made, the pressure of a gas extrapolates to zero when the temperature is -273.15°C. Absolute zero on the Celsius scale is therefore -273.15°C.
These are for isobaric thermodynamic systems (constant pressure). |
|
|
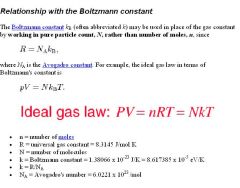
What is Avogadro's gas law?
|

Avogadro's law: Equal volumes of two gases will also contain equal number of mols of each gas (given ideal conditions: ideal gas at STP).
PV = nRT R is constant, and at STP, pressure and temperature is also constant. V/n = RT/P If you plug in STP values, you'll end up with V/n = 22.4 L/mol. All ideal gases at STP will occupy 22.4 L per mol of gas molecules. REMEMBER: STP means 0° C (not 25 degrees Celsius), so it is 273 K!!!!! It is also at 1 atm. But 0 DEGREES CELSIUS IS THE TEMPERATURE FOR STP! Notice that the ideal gas law does not change for different gases behaving ideally (of course not, it's written for an ideal gas). This means that all gases (behaving ideally) will have the same volume, if they have the same temperature, pressure, and number of molecules). At STP, one mole of any gas (behaving ideally) will occupy the standard molar volume of 22.4 liters. |
|
|

How can you compare the average speeds of two gases at the same temperature?
|

You use Graham's law:
molar mass of 1 gas * (average speed of that gas)^2 = molar mass of other gas * (average speed of that gas)^2. This means that M1/M2 = v2^2/v1^2. Based on Graham's law of effusion, larger masses take a longer time to effuse (they have a lower average speed) than smaller masses (they have a higher average speed and effuse out quick). Diffusion and effusion are inversely proportional to square root of mass. If you are given a an unknown gas that is a certain multiple of the speed of the known gas (say take 40% longer to diffuse), and you need to find the empirical formula of the unknown gas, then you would say that the speed of the unknown gas is 1.4 times the speed of the known gas. By squaring the square root of the mass and the speed, we would get (speed)^2 = mass. So (1.4)^2 of the known gas = about 2 times the mass of the known gas. So we would go to the periodic table, multiply 2 times the mass of the known gas, and then find the corresponding empirical formula. Once you know the relative speeds, you can calculate the relative distances that they will travel because say the ratio is: M1/M2 = v2^2/v1^2 and the square root of M1/the square root of M2 is 4:6, that means that the average speeds of v2 to v1 is 4:6, so the speed of M1/M2 is 6:4, so if you have a 1 meter distance, M1 will travel 0.6m of the meter and M2 will travel 0.4 of the meter. Gas will only effuse if there is low pressure in the area that it is effusing to (concentration gradient). Diffusion is the mixture of gases (gases moving through one another). |
Unlike liquids, all gases are miscible with each other; they mix regardless of polarity differences. However, given time and low temperatures, heavier gases tend to settle below lighter gases because of gravity. For instance, liquid gasoline and liquid water don't mix because gasoline is nonpolar while water is polar, however water and gasoline vapors form a homogenous mixture. Hot air rises because it is less dense than cold air. The velocity for Graham's law can be used for rms velocities and for average speeds for gases. Since temperature dictates the average kinetic energy of the molecules in a gas, the gas molecules of each gas in any gaseous mixture must have the same kinetic energy at that temperature. However, the gases have different masses, so they have different rms velocities. By setting their kinetic energies equal to each other, Graham's law shows the relationship between the rms velocities and the masses.
|
|

What is the Kinetic theory of gases?
|

Pressure of a gas is due to its molecules constantly colliding with the walls of its container. At the same temperature and volume, the same numbers of moles of all gases exert the same pressure on the walls of their containers. For two gases, at the same temperature, molar mass of one gas times the average speed of that gas is equal to the molar mass of the other gas times the average speed of that gas. Pressure is equally distributed over the walls of the container because molecular motion is random. Temperature is a measure of the average kinetic energy of the gas molecules. Higher temperature means the molecules are traveling faster, lower temperatures means slower molecules. The average speed of a gas molecular is inversely proportional to it's molar mass - so gas molecules with lower molar masses move at higher speeds than gas molecules with higher molar masses. So the point is, for 2 gases, if the temperature is the same, then the kinetic energy of the gases is the same, and the gases exert the same pressure on the walls of their containers. What is different is the average speed - the average speed depends on the molar mass of the gases - a gas with a lower molar mass will have a faster speed, while the gas with the higher molar mass will have a slower speed. I don't have to memorize the actual molecular speed equation, just know the relationships between the speeds of the gases.
|
If a force is applied to a gas in a container, and the gas's volume is reduced, the temperature of the gas will increase because of the internal energy. The force does work on the gas, which meanst he internal energy of the gas is increased. Since the internal energy of the gas is increased, the temperature, which is average kinetic energy per mole, also increases. So if energy is applied on a gas, the temperature increases.
|
|

What is the qualitative deviation of real-gas behavior from ideal gas law?
|

When molecules are far apart (under conditions of low P, high T), they are ideal. When molecules are brought close together (higher P, lower T and lower volume), they experience intermolecular attraction. At really high pressure, the volume decreases, and the deviation from ideal behavior will be dominated by to molecular volume over intermolecular attractions because V will be higher than the ideal V. When molecules are brought so close together that they clash into one another, they experience steric repulsion. For instance, if the volume of a container is decreased at constant temperature and the gases behave less ideally, then what would be pressure of the gases be in comparison to the ideal gas? The pressure would be lower than the ideal gas because as the volume of the container is decreased, the molecules in the container approach each other more frequently, which leads to an increase in the number and strength of intermolecular attractions among the molecules. As these attractions increase, the number and intensity of collisions decrease, because the collisions are not elastic (where KE and momentum are conserved), they are now more inelastic (where only momentum is conserved and KE is lost) - so the number and energy of collisions against the container decreases (number is decreasing because the molecules are clumping together instead of separating), resulting in a lower pressure. When temperature is lowered at constant volume, the kinetic energy is lower and the gas is travelling at slower speeds. Since it's travelling at slower speeds, the intermolecular attractions increase because the gas molecules are more in contact with each other. The expected difference between using the ideal gas law to calculate pressure and using Van der Waals will depend on the real volume and temperature, as well as a and b. You can't just figure out the difference with just a and b.
|
When molecules are close together, the volume of the molecules become significant compared to the volume around the molecules. Also, as can be seen by Coulomb's law (F = kqq/r^2, when molecules are close together, the electrostatic forces increase and become significant. Gases generally deviate from ideal behavior at pressures above 10 atm and temperatures near their boiling points. Volume decreases with either decreasing pressure or decreasing temperature and getting molecules close together is what causes the deviations in ideal behavior.
|
|

What is the quantitative devation of real-gas behavior from ideal gas law (van der Waals equation)?
|

The variable b is a measure of the actual volume occupied by a mole of gas. The variable a reflects the strength of the intermolecular attractions. b for bounce. The term with the constant b is the repulsion term. The greater b is, the more repulsion, which leads to greater pressure.
a for attraction. The term with the constant a is the attraction term. The greater a is, the more attraction, which leads to less pressure. The values of a and b are constants for specific gases and generally increase with the molecular mass and molecular complexity of a gas. |
Since molecules of a real gas do have volume, their volume must be added to the ideal volume. Thus volume of a real gas > volume of an ideal gas. Second, molecules of a real gas do exhibit forces on each other, and those forces are attractive when the molecules are far apart. In a gas, repulsive forces are only significant during molecular collisions or near collisions. Sinec the predominant intermolecular forces in a gas are attractive, gas molecules are pulled inward toward the center of the gas, and slow before colliding with container walls. Having been slightly slowed, they strike the container wall with less force than predicted by the kinetic molecular theory. Thus a real gas exerts less pressure than predicted by the ideal gas law: Pressure of a real gas < Pressure of an ideal gas.
|
|
|
What is Partial pressure?
|
Partial pressure = a component of the total pressure exerted by a species in a gas mixture.
The total pressure of a mixture of gas = The sum of all the partial pressures. As well as pure gases, we can apply the kinetic molecular theory to mixtures of gases. In a mixture of gases, each gas contributes to the pressure in the same proportion as it contributes to the number of molecules of the gas. This makes sense, given the kinetic molecular theory, because molecules have no volume, no interactive forces other than collisions, and kinetic energy is conserved when they collide. Thus, each gas in a mixture essentially behaves as if it were in its container alone. Thus, the amount of pressure contributed by any gas in a gaseous mixture is called the partial pressure of that gas. The partial pressure of a particular gas is the total pressure of the gaseous mixture times the mole fraction of the particular gas. This is different from finding Raoult's law of vapor pressure lowering because Raoult's law is the vapor pressure lowering is found by multiplying the mole fraction of the solvent times the vapor pressure of the pure solvent. |
Dalton's law states that the total pressure exerted by a gaseous mixture is the sum of the partial pressures of each of its gases. Dalton's law is a good way to understand an ideal gas. Each gas behaves like it is in the container by itself so all of the partial pressures added together equal the total pressure.
|
|
|
What is mole fraction?
|
Mole fraction = a component (fraction) of the total # mols that belongs to a species in a gas mixture.
Mole fraction for species A = # mols of A / # mols of the entire gas mixture. = # mols of A / Σ # mols of A, B, C ... Dalton's law relates partial pressure to mole fraction - the mole fraction is the ratio of partial pressure of the species to the total pressure. Mole fraction is a ratio, so you might have molarity/molarity or (mol/L)/(mol/L). The units will cancel out. Moles could be calculated multiple ways (from molarity values, from molar mass, from calculating molarity first - such as concentration (g/L) and molar mass (g/mol), then dividing the molarity values out. The mole fraction, X, of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution. So, instead of using moles alone, we could calculate mole fraction from molarity (mol/L). |
|
|
|
How does Dalton's law relate partial pressure to composition?
|
Pi = χi·Ptotal
Ptotal = ΣPi = Σχi·Ptotal Ptotal is total pressure. Pi is partial pressure of species i. χi is the mole fraction of species i. The mole fraction is the ratio of the partial pressure of the species to the total pressure. So to get partial presure of a species, you multiply the mole fraction by the total pressure. If you are only given the partial pressure at STP of an ideal gas and you are asked to find the mass, you can't calculate it with that information. Why? Because the equation is PV = nRT and we only have P, R, and T. We don't have V or n. Although we know 1 mole of gas occupies 22.4 L, we don't know what the total volume is of the gas. So, that is not enough info. If we are given the volume, then we can calculate the number of moles, which would then help use to calculate the mass, but that's it. Mole fraction helps us to find partial pressures and % compositions, but it doesn't give the actual number of moles or the actual amount of mass unless we are given the total amount of moles or total amount of mass. If we are given the total amount of moles or mass, then we multiply that number by the mole fraction to get the individual number of moles or mass of the species. |
|
|
|
What are hydrogen bond donors and hydrogen bond acceptors?
|
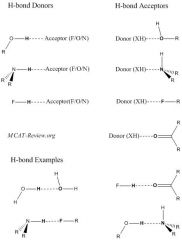
|
|
|
|
What is hydrogen bonding?
|
Hydrogen bonding is a weak interaction between a partially positive H and a partially negative atom.
Technically, hydrogen bonds are a special type of dipole-dipole interaction. Hydrogen bonding increases the boiling point. Partially positive H are also called hydrogen bond donors. They are hydrogens that are bonded to either F, O, or N. Partially negative atoms are also called hydrogen bond acceptors. They are most commonly F, O, or N. Do ethers form hydrogen bonds with other ethers? Ans: no, because ethers do not have a partially positive H (donor). The more polar a bond is, the stronger the hydrogen bond. The H-F bond is the most polar, followed by the H-O bond, and lastly the H-N bond. Hydrogen bonding does NOT occur with Cl (CHLORIDE) because the Cl is NOT electronegative enough. It just happens with N, O, and F. Hydrogen forms a covalent bond with Cl (a dipole-dipole bond). |
|
|
|
What are dipole-dipole interactions?
|

All polar molecules exhibit dipole-dipole interactions. This is where the polar molecules align such that opposites attract. This is an intermolecular force - force between molecules. These forces do not include hydrogen - that is what hydrogen bonding is for. These poles usually involve carbon as the positive pole and a more electronegative atom as the negative pole.
Dipole-dipole interactions increase the boiling point, though not as significantly as hydrogen bonding. Dipole interactions are stronger the more polar the molecule is. Ion-dipole interactions are similar to dipole-dipole interactions, but it's stronger because it is no longer an interaction involving just partial charges. Instead, it is an interaction between a full charge (ion) and a partial charge (dipole). Ion-dipole interactions get stronger when you have larger charge magnitude of the ion, and large polarity of the dipole molecule. |
A polar solute interacts strongly with a polar solvent by tearing the solvent-solvent bonds apart and forming solvent-solute bonds. A nonpolar solute does not have enough charge separation to interact effectively with a polar solvent, and thus cannot intersperse itself within the solvent. A nonpolar solute can, however, tear apart the weak bonds of a non-polar solvent.
|
|

What are London dispersion forces (Van der Waals' forces)?
|

Dispersion forces exists for all molecules, but are only significant for non-polar molecules. For polar molecules, dipole forces are predominant.
Dispersion forces result from induced and instantaneous dipoles. Induced dipoles: when a polar molecule interacts with a non-polar molecule, then polar molecule induces a dipole in the non-polar molecule. Instantaneous dipoles: Non-polar molecules have randomly fluctuating dipoles that tend to align with one another from one instant to the next. Dispersion forces get stronger for larger molecules. For example, decane (C10H22) has a stronger dispersion force than ethane (C2H6). Disperson forces therefore get stronger for molecules with a higher molecular weight, because there are more electrons and those electrons have a greater ability to produce the temporary dipoles (polarize). |
|
|
Explain the phases of solid, liquid, and gas.
|
Solid: atoms/molecules vibrate about a fixed position. Hard to compress. Does not flow to fill a container.
Liquid: atoms/molecules move about, but are close together and bound by intermolecular forces. Hard to compress. Flows to fill a container. Gas: atoms/molecules fly about far apart from one another and do not experience intermolecular forces. Easy to compress. Flows to fill a container. Liquids and solids are hard to compress, gases are the only form of a substance that is easy to compress. When you have a liquid, the atoms have a certain level of attraction between each other due to the electrostatic potential. In order for the liquid to turn into a gas, it requires energy input to be great enough that the kinetic energy is greater than the potential energy and it escapes from the attractive force and becomes a gas - that is what occurs during boiling. |
If all the intensive macroscopic properties of a system are constant, that system is said to be homogenous. Any part of a system that is homogenous is called a phase. A phase is uniform throughout with respect to chemical composition and physical state. A system may have a number of solid or liquid phases, but it will usually only have one gaseous phase. Pure substances have only one gaseous phase and usually have only one liquid phase. Most of the time, you can think of phases as solid, liquid, and gas, but this isn't the technical definition. Pure water is a different phase than aqueous Na+ ions. Rhombic sulfur and monoclinic sulfer are 2 different solid phases of the same element.
|
|
|
Explain the phase diagram.
|
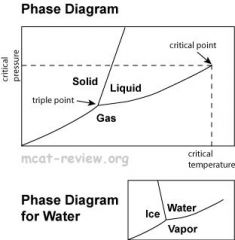
Solid-liquid boundary: solid and liquid exist in equilibrium.
Solid-gas boundary: solid and gas exist in equilibrium. Liquid-gas boundary: liquid and gas exist in equilibrium. Triple point: the temperature and pressure at which all three phases of matter coexist in an equilibrium. Critical point: At a temperature above the critical temperature, Tc, and a pressure above the critical pressure, Pc, it is no longer possible to distinguish between the gas and liquid phases. At T > Tc and P > Pc the substance is referred to as a super-critical fluid. Water phase diagram is different from others because the solid-liquid boundary has a negative slope. This is because water (liquid) is more dense than ice (solid). Increasing the pressure drives the ice into the higher density phase, which causes melting. A superheated liquid is a liquid heated above it's boiling point. |
Most substances can be classified as either a solid or a fluid. The molecules of a solid are held in place by molecular bonds that can permanently resist a force from any direction. A fluid is a liquid or gas. Unlike a solid, any existing molecular bonds in a fluid are constantly breaking and reforming due to the high kinetic energy of the molecules. Since the molecules of a fluid are not arranged with any order or structure, but move about in random directions relative to each other, a fluid has only temporal (impermanent) resistance to forces that are not perpendicular to its surface. However, since fluid molecules require room to move collectively they can
create a permanent force outward from within the fluid. The only permanent force that a resting fluid can exert is one normal to its surface. Each phase of a substance has a unique slope andhas it's own specific heat, because the slope = 1/(mc), where c is the specific heat and m is the mass. During phase changes, the heat breaks bonds, but does not change temperature. When the phase is NOT changing, energy increases molecular movement, which increases temperature. Above the critical point, liquid and vapor water have the same density. |
|
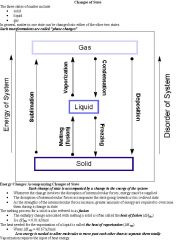
How do you calculate the total enthalpy change for converting ice from -25° C to 125° C?
|

Heat flow can either perform enthalpy change (at constant pressure) or can perform work (at constant temperature).
Basically, the equation for enthalpy, ΔH = ΔU + PΔV, means that enthalpy can change from a change in the internal energy (ΔU = q+w) or a change in the work (PΔV). Enthalpy is also used to calculate specific heat c at constant pressure. ΔH = c*ΔT. T is in kelvins. So if you know two enthalpy values and two temperature values, you can calculate the specific heat from the changes between the two. |
Although enthalpy is measured in units of energy (joules), enthalpy itself is not conserved like energy. Enthalpy of the universe does not remain constant. Due to the close relationship between internal energy and enthalpy, the term energy is used loosely for energy graphs. The y axis may be labeled as enthalpy, Gibbs free energy, or simply energy. If you are asked to find the enthalpy of reaction, it's easier to use: ΔH of reaction = ΔH of products - ΔH of reactants. If you are given the ΔH of formation of the compounds and you are asked to find the ΔH of the reaction, then you don't need to write out the formation equations and switch ΔH signs. You just need to make sure to multiply the ΔH's by the respective number of moles and remember that molecules in their elemental forms is has ΔH = 0 at 298K (25°C) (like diatomic oxygen), so they are not included in the products or reactants equation. And make sure to sum the products up and sum the reactants up first before subtracting them from each other. If you are given ΔH of some reactions, and are asked to find the ΔH of a new reaction, then you need to sum up the reactions together and switch ΔH signs, if necessary, and also multiply by the number of moles. We still don't need to write out the ΔH of formation reaction, but we may need to reverse the reactions and the ΔH signs that we are given to create the new reaction. Then we add the ΔH's up. Internal energy, pressure and volume are the properties of a gaseous system (ΔH = ΔU + PΔV). Enthalpy depends on temperature for an ideal gas. Ideal gases and gaseous systems are two different things.
|
|

What is Freezing point, melting point, boiling point, and condensation point?
|

Freezing point: temperature (at a given pressure) that liquids begins to freeze into a solid. Melting point: temperature (at a given pressure) that a solid begins to melt into a liquid. Boiling point: temperature (at a given pressure) that a liquid begins to turn into a gas. Condensation point: temperature (at a given pressure) that a gas begins to condense into a liquid. Freezing point and melting point are the same, they can both be found along the solid-liquid phase boundary. Boiling point and condensation point are the same, they can be found along the liquid-gas boundary. Sublimation: conversion of a solid directly into a gas. Conditions for sublimation can be found along the solid-gas boundary. Melting point and boiling point can be thought of as a progressive weakening of the forces between the molecules. Anything that strengthens the forces between the molecules makes it harder to melt (increases the melting point) or harder to boil (increases the boiling point). Anything that weakens the forces between the molecules (like with branching of atoms in a molecule) makes it easier to melt because the forces between the bonds break up easier, so it lowers the melting point. Normal boiling point of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, 1 atmosphere. Water boils when the vapor pressure equals the atmospheric pressure, at 100° C and 1 atm or 760 torr. Since the vapor pressure increases with temperature, it follows that for pressure greater than 760 mmHg (e.g., in a pressure cooker), the boiling point is above 100°C and for pressure less than 760 mmHg (e.g., at altitudes above sea level), the boiling point will be lower than 100°C. Condensation is also when the partial pressure of the liquid in the atmosphere is at least as great as the vapor pressure of the liquid. Why? Because it follows Henry's law. If the partial pressure of the gas above the liquid is greater than the gas dissolved in the liquid (for example the vapor in the liquid versus the partial pressure of the water in the atmosphere), then more of the water in the atmosphere will dissolve into the liquid. If the partial pressure of the water in the atmosphere is lower than the vapor pressure in the liquid, then more water will exit the liquid as gas, causing evaporation. Boiling is when the vapor pressure of the liquid equals the TOTAL pressure of the atmosphere, not a PART of the pressure of the atmosphere.
|
|
|
|
What is molality?
|
Molality is a measure of the concentration of solutes in a solution.
Molality is given the symbol m (don't confuse the small case m with the large case M that is molarity) Molality = mols of solute / mass (in kg) of solvent. Molality (mol/kg of solvent) is not the same thing as molarity (mol/L of solution). The difference in finding something in molality versus in molarity is that for molarity, you would first measure the grams of the solute that you plan to use, put it in a flask, and then add water until it is the right concetration. This is because molarity is L of SOLUTION (solvent + solute). Molality is different because you would first measure the solvent before you add it to the the solute because it is kg of SOLVENT. For solutions with water as a solvent, molarity and molality are almost (but not exactly) the same because 1 L of water weighs around 1 kg. |
Ideal solutions are solutions made from compounds that have similar properties. The compounds can be interchanged within the solution without changing the spatial arrangement of the molecules or the intermolecular attractions. In an ideally dilute solution, the solute molecules are completely separated by solvent molecules so that they have no interaction with each other. Nonideal solutions violate both of these conditions. One the MCAT, assume ideally dilute solutions, which have a mole fraction of the solvent of about 1. But don't assume an ideal solution.
|
|
|
What are colligative properties?
|
Colligative properties are properties of solutions that depend on the number of molecules in a given volume of solvent and not on the properties (e.g. size or mass) of the molecules. Colligative properties include: lowering of vapor pressure; elevation of boiling point; depression of freezing point and osmotic pressure.
Solute particles in solution likes to keep the solution in liquid phase. This is why it makes it harder to boil (raises its boiling point) and also makes it harder to freeze (lowers the freezing point). Lowering the vapor pressure is just another fancy name for raising the boiling point. If you have two solutions, the one with the greater number of solute particles per liter of solution will freeze at a lower temperature. Solutions are homogenous mixtures like ions dissolved in water, water vapor in gas, and alloys are also solutions. Vapor pressure is independent of atmospheric pressure, but boiling point and evaporation are not independent. We know that vapor pressure is independent of atmospheric because of Raoult's law. Change in vapor pressure of the solvent is equal to the mole fraction of the nonvolatile solute times the vapor pressure of the pure solvent. Stirring solutions creates a homogenous mixture and uniform distribution of temperature, both of which makes up a single phase. |
|
|

What is vapor pressure lowering (Raoult's law)?
|

Consequently, as the number of components in a solution increases, the individual vapor pressures decrease, since the mole fraction of each component decreases with each additional component. If a pure solute which has zero vapor pressure (it will not evaporate) is dissolved in a solvent, the vapor pressure of the final solution will be lower than that of the pure solvent. Entropy increases with solvation of a solution (mixing of a solution). A way to determine vapor pressure of a pure liquid is to allow the liquid to reach equilibrium with its vapor pressure and measure the partial pressure of the vapor. Evaporation and boiling points are two different processes. Evaporation is a type of vaporization of a liquid, that occurs only on the surface of a liquid. The other type of vaporization is boiling, that instead occurs on the entire mass of the liquid. The rate at which the liquid (or solid) turns into vapor must be greater than the rate at which the vapor turns into solid. So the vapor pressure of the liquid or solid must be greater than the partial pressure of its vapor above it. However, with boiling, the vapor pressure of the liquid must be greater than the TOTAL pressure of the gas above it. A lot of times, this TOTAL pressure is atmospheric pressure. Boiling point is the temperature above which liquid can no longer exist at equilibrium, but vapor can exist below the boiling point, allowing for evaporation and condensation processes. Vapor pressure always increases with temperature. Solids and liquids can exist in equilibrium with their vapor pressures regardless of pressure or temperature.
Raoult's law says: The vapour pressure of a solution of a non-volatile solute is equal to the vapour pressure of the pure SOLVENT at that temperature multiplied the mole fraction of the SOLVENT. it applies to solutions in which the solute is non-volatile, meaning the solute does not have vapor pressure. |
Equilibrium between the liquid and gas phases of a compound occurs when the molecules move from liquid to gas as quickly as they move from gas to liquid. The vapor pressure necessary to bring the liquid and gas phases of a compound to equilibrium is called the vapor pressure of the compound. Since vapor pressure is related to the kinetic energy of the molecules, vapor pressure is a function of temperature. Vapor pressure increases with temperature. When you have volatile solutes, you can still use Raoult's law. You would apply Raoult's law separately to the solvent and the solute. Remember, solutes don't necessarily mean "solids", it could be liquids or gases. It is the part of the solution that is less in quantity than the solvent. Since it has vapor pressure, it has to be counted and you would multipy the mole fraction of the solute times the vapor pressure of the pure solute.
|
|
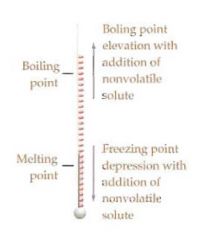
What is freezing point depression?
|
ΔTf = (-kf)(m)(i)
ΔTf is the decrease in freezing point (the negative sign shows that the change is a decrease). kf is the molal freezing point constant. m is the molality (mol/kg). i is the Van't hoff factor The freezing point of water and the melting point of ice is 0° C at 1 atm. The boiling point of water is 100° C at 1 atm. An increase of 1° C is equivalent to an increase of 1 K. The lowest possible temperature is absolute zero and is approximately -273° C. This equation is the freezing point depression for an ideally dilute solution with a nonvolatile solute. Because freezing point is lowered, melting point is lowered. |
Be careful with freezing point depression because it's factor is CRYSTALLIZATION. If you add a liquid solute, the impurities will initially lower the melting point (making it easier to freeze). However, as the mole fraction of the solute increases, the solvent and solute interact in a way that makes it harder to freeze, so the freezing point rises.
|
|
|
What is osmotic pressure?
|
Osmotic pressure determines whether and in what direction osmosis will occur.
Osmosis is the movement of solvent across a semi-permeable membrane from an area of low solute concentration (high solvent concentration) to an area of high solute concentration (low solvent concentration). Solvent will move from an area with high π value to an area with low π value. Osmotic potential is a partial measure of a system's free energy. Pure water is arbitrarily assigned an osmotic potential of zero. When solute is added, the osmotic potential becomes negative. At constant temperature and pressure, water flows from higher osmotic potential to lower osmotic potential. Water potential, is similar to osmotic potential, but takes into account temperature and pressure. Water potential is the same as free energy. |
Think about osmotic pressure as the pressure pulling into a solution and hydrostatic pressure as the pressure pushing out of a solution. Just remember that pressure is scalar and have no direction, but this gives an intuitive sense of it.
|
|
|
What are colloids?
|
Solution: things are mixed at the molecular level and will always stay mixed. When you use the term dissolve, you are making a solution.
Colloids: things are mixed at a "semi-molecular level" with solute aggregates that are really really tiny. Colloids will stay mixed until you centrifuge it. Suspension: things are mixed at a particle level and will NOT stay mixed. The famous colloid example is milk. Also, when you shake water and oil vigorously, you can get an emulsion, which is a colloid. An emulsion is a mixture of two or more immiscible (unblendable) liquids. Colloids are too small to be extracted by simple filtration. |
A colloid is like a solution, only the solute particles are larger. The colloid particles are usually too small to be extracted by filtration but usually large enough or charged enough to be separated by a semipermeable membrane. Heating a colloid or adding an electrolyte may cause the particles to coagulate. The larger particles produced by coagulation will settle out or can be extracted by filtration. Separating by a semipermeable membrane is a process called dialysis (used by kidneys to separate the blood plasma, a colloid).
|
|
|
What is molecular weight?
|
Molecular weight = mass of 1 mol of that substance = g/mol = grams per mole
1 g/mol = 1 amu 12Carbon has 12 amu and weighs 12 g/mol Calculate molecular weight of a substance from the atomic weights given in a periodic table as either amu or g/mol. The atomic weight of an elementi s actually a mass and not a weight. ONE atom of 12C has an atomic weight of 12 AMU. Or put in another way 1/12 the 12C atom is 1 AMU. All other atomic weights are measured against this standard. 12C also defines a mole. A mole (or Avogadro's number, 6.022x10^23) is the number of carbon atoms in 12 grams of 12C. 6.022x10^23 amu = 1 gram. Moles = grams/ (atomic or molecular weight) |
|
|
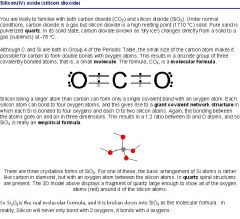
What is the difference between empirical formula and molecular formula?
|
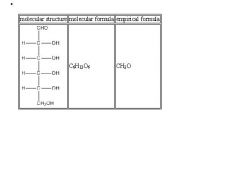
Empirical formula is what you get after dividing everything in the molecular formula by the highest common factor. The empirical formula of a chemical compound is the simplest whole number ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. In contrast, the molecular formula identifies the number of each type of atom in a molecule. When comparing items in a chart and you are asked to show the best effect of 1 thing, like the best effect of molecular weight alone on melting point, or the best effect of structure alone on melting point, and you have a chart with molecular weight, structure, and anything else, try to pick the 2 molecules that have most similar aspects of everything except what you're trying to compare, here, it is the melting point. So if the 2 molecules have very different structures and molecular weights, they won't be good at comparing to find the effect of melting point because there are 2 confounding issues.
|
A substance made from two or more elements in definite proportions is called a compound. An empirical formula may not tell you how many atoms each atom is bonded to in actuality. For example, SiO2 is the empirical formula for silicon dioxide, but silicon actually bonds to 4 oxygens and each oxygen bonds to 2 silicons.
|
|

What are the metric units commonly used in the context of chemistry?
|
Molarity = M = mol/L
molality = m = mol/kg mass = kg. molar mass = g/mol. Molar mass could be of a particular element, or of a molecular species. If it is of molecular species, then we can calculate the molar mass of the species in a reaction by dividing the grams of species in the reaction by the number of moles of the species in the reaction. Then, we figure out what the species could be by adding up the molar masses of elements to get the total molar mass of the species. Mole fraction is not affected by temperature. The mole fraction of a solution is the number of moles of solute divided by the number of moles of solution, and temperature changes do not change the number of moles in a sample. Since density changes with temperature, the volume of a solution can change when the temperature changes - so molarity is affected by temperature. Molality is not affected by temperature because moles and kg are not affected by temperature. Molarity and Molality are used to measure concentration, not mole fractions. If you are asked to find the concentration of ions in aqueous solution (like chloride ions in NaCl mixed with water), pay attention to how the salt dissolves. Since each NaCl dissociates into one chloride ion and a sodium ion, the molarity of the chloride is the same as the molarity of the NaCl solution. |
Pico is 1x 10^12 and femto is 1 x 10^-15. -15 (fifteen) - is f for femto.
Remember that solution concentrations are always given in terms of the form of solute BEFORE dissolution. So if 1 mole of NaCl is added to 1 litter of water, it is approximately 1 molar solution of NaCl and NOT a 2 molar solution even though NaCl dissociates into 2 ions. |
|

What is the description of composition by percent mass
|
%mass = mass of species of interest / total mass * 100
If you are given the partial pressures of a gas, and you know the total pressure (say the partial pressures are at STP), then you can calculate the mole fraction (because it would be the partial pressure of the gas/standard pressure). But if you don't know the total number of moles and you are asked to calculate the percent by mass, then you can use any number as the total number of moles since we're trying to find grams. So, say the sample has a total of 100 moles of gas, that would make it an easier calculation. Then, find the mole fraction of each gas and multiply it by 100 moles. Then, use that number of moles of the gas and multiply it by its molar mass (g/mol). Voila! Now you have the number of grams (because we want % by mass). So, you add up the number of grams and use that as the denominator (total number of grams), and then you put whatever species we want the % by mass of and put that on the numerator (mass of species of interest). If you work out a problem with percent mass, and you come up with all fractions for the number of moles, divide the fractions by the lowest fraction of the moles. That will give you the final molar ratio of each element. So for instance, if you calculat 3.6 moles for O, 1.2 moles for S, and 2.4 moles for H, then divide all the moles by 1.2 and you get 3 moles for O, 1 moles for S and 2 moles for H for the final equation. Sometimes rounding will work against you in this process, so look out for this. |
|
|
|
What is the mole concept or Avogadro's number?
|
1 mole = 1 mol = 1 Avogadro's number = 6.02E23 molecules
The mole is the fundamental unit in the SI system and it is used to measure the amount of a substance (or chemical amount). The mole is to the amount of a substance as the gram is to mass. In Latin, mole means a massive heap of material, and it's convenient to think of 1 mole as such. |
|
|
|
What is the definition of density?
|
density = mass / volume = kg/m3
often in chemistry, specific gravity is used. specific gravity = number of times the density of water = density of substance / density of water density of water = 1 g/mL = 1 g/cm3 specific gravity of water = 1 g/cm3 / 1 g/cm3 = 1 density of lead = 11 g/cm3 specific gravity of lead = 11 g/cm3 / 1 g/cm3 = 11 specific gravity is unitless |
Notice that a specific gravity of less than one indicates a substance lighter than water; a specific gravity of one indicates a substance equally as heavy as water; a specific gravity greater than one indicates a substance heavier than water. Since we
al1 have an intuitive feel for the heaviness of water we can relate this to a substance, if we know its specific gravity. The specific gravity of mercury is 13.6, so lifting one bucket of mercury would be equivalent to lifting 13.6 buckets of water. |
|
|
What are the common oxidizing and reducing agents?
|

|
|
|
|
What are disproportionation reactions?
|
An element in a single oxidation state reacts to form 2 different oxidation states.
Disproportionation can occur when a species undergo both oxidation and reduction. For example: 2Cu+ → Cu + Cu2+ Here, the Cu+ acts as both oxidizing and reducing agent and simultaneously reduce and oxidize itself. The oxidized Cu+ becomes Cu2+ The reduced Cu+ becomes Cu |
|
|
|
How is Iodine used in redox titrations?
|
Iodine is used in redox titrations because in the presence of starch, I2 is dark blue while I- is colorless.
You can only accurately titrate something going from dark to colorless ( I2 → 2I-), but not the otherway round. A redox titration does not necessarily need the presence of Iodine. As long as some type of color change can be seen at the equivalence point of the redox reaction, then it will work. For example: 5 H2O2 + 6 H+ + 2 MnO4- → 5 O2 + 2 Mn2+ + 8 H2O Goes from purple to colorless because of MnO4- → Mn2+ transition. Redox titrations are similar to acid-base titrations, except instead of measuring pH, you look for a color change. |
|
|

What are the conventions for writing chemical equations?
|
Phases
(s) = solid (l) = liquid (g) = gas (aq) = aqueous (dissolved in water) Coefficient -an equation with coefficients is a balanced equation. Direction - A single head arrow denotes the reaction goes to completion in the direction of the arrow. A double-sided arrow denotes a reaction in equilibrium. A double-sided arrow with one side larger than the other denotes an equilibrium in favor of the side of the larger arrow. Charge - Denotes charge and magnitude, for example +, -, 2+, 5- ...etc. Neutral charges are not denoted. Pure liquids and solids do not enter equilibrium expressions. Reactants and products in pure liquids and solid phases generally have an exponent of zero, so they are not included in the equilibrium expression. |
When a compound undergoes a reaction and maintains its molecular structure and thus its identity, the reaction is called a physical reaction. Melting, evaporation, dissolution, and rotation of polarized light are physical reactions. When a compound undergoes a reaction and changes its molecular structure to form a new compound, the reaction is called a chemical reaction. Combustion, metathesis, and redox are chemical reactions. Metathesis occurs when substituents are exchanged in a chemical reaction.
|
|
|
Balance the combustion of propanol: C3H8O + O2 → CO2 + H2O
|
Pick out the atom (or group) that is the easiest to balance (usually represented in only 1 term on both side of the equation. In this case it is carbon.
C3H8O + O2 → 3CO2 + H2O The next easiest to balance is hydrogen C3H8O + O2 → 3CO2 + 4H2O Leave the hardest to last, oxygen. O is present in every term of the equation, so if we tried to balance O first, we'd be having a hard time. However, now that we balanced every other term, this leaves only one term left that contains O and that we haven't balanced yet. Do a quick count of oxygen atoms: there's 1 from C3H8O, 3x2 from 3CO2, and 4x1 from 4H2O. Set up this equation: 1 + 2x = 3x2 + 4x1, where x would be the coefficient of our last term, O2. Solve for x C3H8O + 9/2O2 → 3CO2 + 4H2O Even though we balanced out every term, we're not done yet. We need to get rid of any fractions, so multiply every term by 2. 2C3H8O + 9O2 → 6CO2 + 8H2O |
|
|
|
What are limiting reactants (reagent)?
|
Limiting reactant is the reactant that will get all used up first. The limiting reactant determines the amount of product that will be produced. It doesn't matter how much other reactant you add (if the reaction isn't at equilibrium), the limiting reactant determines the amount of product produced.
What is the limiting reactant for the following reaction? 3Xox + Ared → 3Xred + Aox Given: You use 60 grams of Xox and 63 grams of Ared Given: the molecular weight of Xox is 2 amu, and Ared is 7 amu. The first thing you do is convert everything in moles. 1 amu = 1 g/mol. Xox: 60 g / 2 amu = 30 mols. Ared: 63 g / 7 amu = 9 mols. Now here's where stoichiometry comes in: divide the mols by the stoichiometric coefficient of the species: 30 mols / 3 = 10 for Xox 9 mols / 1 = 9 for Ared Now compare the values. 9 is the smallest, so Ared is the limiting reactant. Limiting reactant can also be called the limiting reagent, limiting species, limiting [whatever]. A reaction stops when one of two things has happened, either the system has reached equilibrium or one of the reactants has been consumed completely. Systems in equilibrium don't have a limiting reagent, since an amount of the reactants always remain. Other types of reactions, such as between a strong acid and base, proceed until they are limited by one of the reactants running out. To find the limiting reagent, find the number of moles of the reactants and figure out how much product each of those reactants will make. Then see which one makes the least amount of product. That is the limiting reactant. NOTE: It does not matter which product is chosen, but the same product must be used for both reactants so that the amounts can be compared. |
|
|

What is theoretical yield?
|
The theoretical yield is what how much of the product will be made based on stoichiometry if the reaction ran to completion.
In calculating the theoretical yield, first find out what your limiting reactant is. Then, use your limiting reactant as the stoichiometric basis to calculate how much product you will get. In real life, the experimental yield is always less than the theoretical yield because of loss during steps of the reaction (now you can have a higher experimental yield if you're in a chem lab and you accidentally dumped in more reactants than you realized). Reactions often don't run to completion, and sometimes there are competing reactions that reduce the actual yield. |
|
|

What is a Thermodynamic system?
|

A thermodynamic system is just a fancy name for the system that you are studying.
Isolated system: no exchange of heat, work, or matter with the surroundings. Overall, it's no exchange of matter or energy. Closed system: exchange of heat and work, but not matter with the surroundings. It's an exchange of energy, but not matter. Open system: exchange of heat, work and matter with the surroundings. It's the exchange of energy and matter. Work can be in the form of a force over a distance (W = Fd). Heat exchange can be prevented by insulation, such as by non-moving air or a vacuum. |
|
|

What is a state function?
|

In thermodynamics, a state function, state quantity, or a function of state, is a property of a system that depends only on the current state of the system, not on the history and the way in which the system acquired that state (such as whether heat or work happened). It only depends on the initial and final states. For instance, ΔU = q + w and q and w are path dependent, while ΔU is not. A state function describes the equilibnrium state of a system. For example, internal energy, enthalpy and entropy are state quantities because they describe quantitatively an equilibrium state of thermodynamic systems. In contrast, work and heat are process quantities because they describe quantitatively the transition between equilibrium states of thermodynamic systems. Because work is not a state function, the path that the system takes matters. The change in work has to be calculated from the different paths that it takes. If a system goes from state A to state B and back to state A, you can't say that it did no work. You would calculate the work from state A to the state B, then calculate the work from state B to state A, then add up the work to get the net work.
State functions include: ΔH (enthalpy), ΔS (entropy), ΔG (free energy change), ΔU (internal energy change), temperature, and density. State functions are quantities that can be measured without knowing the history of the system. We cannot calculate H or U, we can only calculate ΔH and ΔU. Entropy of a closed system is always increasing and entropy of the universe is always increasing. So, with a closed system, the gain in entropy must be larger than the lost of entropy, because entropy always increases in a closed system. |
Be familiar with these 7 state functions: Internal energy (U), Temperature (T), Pressure (P), Volume (V), enthalpy (H), entropy (S), and Gibbs energy (G).
|
|

Why is there a difference between the heat of formation of water vapor and the heat of formation of liquid water?
|

The heat of formation of water vapor is negative (exothermic), but is less negative than the heat of formation of water liquid. The difference between the two heat of formations is that there is stored energy in the water vapor that is used to condense into liquid - because in order for a gas to condense into a liquid, it has to release heat. So this stored energy makes the enthalpy less negative (more positive). For heat of formation problems, first write out the equation to see how many moles of the elements would be needed to create the compound, then figure out what the enthalpy of the overall reaction could be. Hydrogen, oxygen, nitrogen, and fluorine are assigned a heat of formation of zero for their diatomic forms because that is when they are most thermodynamically stable. The standard enthalpy of formation of atomic oxygen is positive because atomic oxygen is formed by breaking the oxygen gas bonds. Even though atomic oxygen is an element, the standard enthalpy of formation is not zero. Atomic oxygen is not stable by itself, it has to be formed by breaking up the stable oxygen gas. The standard enthalpy of formation is for stable reactants in their elemental components creating stable compounds, all in their standard states. Gibbs free energy and enthalpy are calculated at constant temperature because we are looking at how heat is being absorbed and evolved. If temperature were changing, it would add energy (kinetic energy) to the system and would change how the reaction would proceed. That is why spontaneous reactions would occur at constant temperature and pressure because constant pressure means there is work being done and constant temperature means heat is being transferred within the system. ΔG is the maximum amount of energy which can be “freed” from the system to perform useful work.
|
|
|

What are enthalpy H and standard heats of reaction and formation?
|

Enthalpy or H is the heat content of a reaction. Mnemonic: H stands for heat. H = U + PV, where U is internal energy. If pressure is constant, ΔH = q.
ΔH is the change in the heat content of a reaction. + means heat is taken up, - means heat is released. Standard heat of reaction, ΔHrxn, is the change in heat content for any reaction. Standard heat of formation, ΔHf, is the change in heat content in a formation reaction. A formation reaction is where a compound or molecule in its standard state is formed from its elemental components in their standard states. The standard state is where things are in their natural, lowest energy, state. For example, oxygen is O2 (diatomic gas) and carbon is C (solid graphite). The heat of formation for oxygen and carbon is 0 because they are already in their standard states. The unit for enthalpy is in energy (J), or it can be expressed as energy per mol (J/mol). When you see ΔHf, for instance ΔHf of HCl = -92.5 kJ/mol, then write the reaction out. ΔHf is the enthalpy of formation of HCl, which means 1/2H2 + 1/2Cl2 yields HCl ΔH = -92.5 kJ/mol. This means that -92.5 kJ/mol is released when H2 and Cl2 come together and form HCl. So if you see another reaction like 2HCl yields H2 and Cl2, then the enthalpy of this reaction is +185 kJ/mol because first of all, HCl is breaking up into H2 and Cl2, the reverse of the enthalpy of formation reaction, so enthalpy is positive. Second, 2HCl has double the moles as HCl, so we have to multiply the enthalpy of HCl by 2. Enthalpy change of reaction is the same thing as the heat of the solution. By equating ΔH = q, we are making heat flow q, a state function. We want to be able to say that nomatter what path we take, as long as the system is at constant pressure, the sum of the heat flow is always the same - this helps to carry out reactions at the benchtop. Controlling pressure is easier than controlling volume. |
|
|
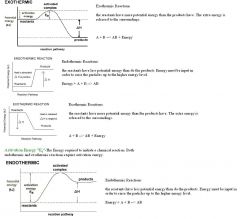
What are endothermic and exothermic reactions?
|
Endothermic = energy is taken up by the reaction in the form of heat. ΔH is positive.
Exothermic = energy is released by the reaction in the form of heat. ΔH is negative. Endothermic and exothermic isn't just based on whether bonds form or break - endothermic and exothermic reactions depend on the potential energy. If there is more potential energy in the reactants than the products, then the extra energy will be released into the surroundings - exothermic. If there is more potential energy in the products than the reactants, then the reactants need energy inputed in order to carry out the reaction - endothermic. The questions should say whether or not a reaction is exothermic or endothermic - we cannot assume a reaction is one way or the other without knowing whether or not energy is absorbed or released. The higher the temperature, the more the solubility. When you add heat, it favors the endothermic reaction. Since solubility increases at higher temperature, such as for salt, means that the dissolution of salt is an endothermic process. |
|
|
|
What is Hess’s law of heat summation?
|
ΔHrxn = ΔHf (products) - ΔHf (reactants)
Hess's Law states that the heat evolved or absorbed in a chemical process is the same whether the process takes place in one or in several steps. In other words, an energy change is path independent, only the initial and final states being of importance. This path independence is true for all state functions. Note that because the standard enthalpies of the elements in their standard states is zero, if you see that there is a difference in coefficients of elements in a balanced equation and you're trying to add the equations together, just disregard those elements (if everything else is balanced). You can disregard them because their enthalpies is zero in their standard states anyway. |
|
|

What is Bond dissociation energy as related to heats of formation?
|

Bond dissociation is the energy required to break BONDS. Bond dissociation energy is the energy required to pull 2 atoms apart, while ionization energy is the energy of pulling a SINGLE ELECTRON from 1 atom. Bond dissociation energy is at the molecular level, while ionization energy is at the atomic level.
ΔHrxn = Bond dissociation energy of all the bonds in reactants - bond dissociation energy of all the bonds in products. If you use bond energies to calculate enthalpy, make sure to do REACTANTS - PRODUCTS, because to break the bonds of reactants, enthalpy is + because energy is absorbed and to form bonds of products , enthalpy is - because energy is released. ΔHrxn = Enthalpy of formation of all the bonds in products - Enthalpy of formation of all the bonds in reactants. Bond dissociation energy is positive because energy input is required to break bonds. The enthalpy of formation of bonds is negative because energy is released when bonds form. With questions, pay attention to the number of moles when calculating enthalpies of reactions - make sure to use Hess's law when needed. Combustion reactions release heat, so the change in enthalpy is always negative (it's an exothermic reaction). |
If we separate the atoms by an infinite distance, the forces between them, and thus the energy, go to zero. The energy necessary to achieve a complete separation is the bond dissociation energy or bond energy. Energy is always required to break a bond. No energy is ever released by breaking a bond. Energy from ATP is released when the new bonds of ADP and iP are formed, and not when the ATP bonds are broken.
|
|
What is calorimetry and what is a calorimeter?
|
Calorimetry is the quantitative measurement of the heat required or evolved during a chemical process.
If you are trying to conserve energy, it would be efficient to use a liquid that has a lower specific heat capacity, because it takes less energy to raise its temperature by a certain amount. A calorimeter is a device which measures energy change. There are constant pressure and constant volume calorimeters. A coffee cup calorimeter is a constant pressure calorimeter because it measures energy change at atmostpheric pressure. Here q = ΔH. A bomb calorimeter measures energy change at constant volume. A bomb calorimeter tells us the internal energy change in a reaction (q = ΔU). We can calculate q for both calorimeters from q = mcΔT and q = CΔT. The heat of the solution is q. |
If you have 2 specific heats and all of the other conditions are the same, if 1 specific heat of A is 1/3 as large as the specific heat of B, then 1/3 as much energy will be required of A to get the same temperature change as B.
|
|

What is heat capacity and specific heat?
|
Heat capacity = the amount of heat required to raise the temperature of something by 1 °C. Heat capacity is: c = q/ΔT. Molar heat capacity = heat capacity per mol = J / mol·°C. Specific heat (capacity) = heat capacity per mass in grams = J / g·°C. Celsius can be replaced by Kelvin here because a change in 1 °C is the same as a change in 1 K. It takes 4.2 J of heat energy to raise the temperature of 1 gram of water by 1 °C. Some useful conversion factors: 1 calorie = 4.2 J; 1 Calorie (with capital C) = 1000 calorie = 4200 J. For water, 1 gram = 1 cubic centimeter = 1 mL. Specific heat of water = 4.184 J/g·K - MEMORIZE! Specific heat of water is also 1 cal/g°C - MEMORIZE!. The amount of heat required to change the temperature of a species (net heat flow q) is dependent on the specific heat of the species,c. The equation is q = mcΔT. The relationship between heat and temperature change is usually expressed in the form shown above where c is the specific heat. The temperature change of a gas is dependent on the number of moles. The specific heat capacity (more properly "mass-specific" heat capacity) is defined as the heat required to raise one unit mass of substance by one degree of temperature. Realize that any formula involving ΔT can use Celcius or K because both will yield the same value when you subtract celcius from celcius or Kelvin from Kelvin. Change in temperature is the same as when measured in Kelvin or degrees celcius. So if a problem gives you the heat that it takes to raise something by 10° C, that same heat would be required to raise something by 10K. Specific heat does not apply if a phase change is encountered, because the heat added or removed during a phase change does not change the temperature. Heat capacity is a measure of how hard it is to change the temperature of a substance. If one substance changes temperature more easily than another substance, it must have a lower specific heat c. Also, the specific heat of water is 1.0 cal/g*K. A high heat capacity means that a liquid gains or loses energy with smaller changes in temperature. The larger and/or more complicated a molecule is, the more likely it is able to absorb more energy vibrationally, rotationally, translationally, and intermolecularly, keeping it's temperature down and increasing it's heat capacity and specific heat. Specific heat is J/g*K while molar heat capacity is J/mol*K.
|
Heat capacity C is a measure of the energy change needed to change the temperature of a substance. Don't let the name 'heat capacity' fool you. Heat is a process of energy transfer and cannot be stored. Assume that heat capacity does not change with temperature. Think of heat capacity of a substance as the amount of energy a substance can absorb per unit of temperature change. If you are given a problem where they want you to calculate the heat of solution in J/mol, you can first calculate the heat of the reaction using the specific heat given in the question. Then, if they give you the number of grams of a substance in the reaction, then you find the moles from that number of grams and divide the number of joules by it. If you are given the specific heat of a reactant, you don't need to find the number of moles of that reactant.
|
|

What is entropy and the relative entropy for gas, liquid, and crystal states?
|

Entropy = measure of disorder = energy / temperature = J / K (it can also be expressed as molar entropy in J / mol·K)
Entropy of gas > liquid > crystal states. At room temperature, the gas molecules are flying around, but the table in front of you is just sitting there. So, gas have more disorder. Reactions that produces more mols of gas have a greater increase in entropy. Positive entropy change (more disorder) is greater than 0. Entropy is an extensive property because it depends on the size of the system. Bond formation is always exothermic and always results in a decrease in entropy because there is less disorder. Spontaneous reactions result in an increase in entropy and entropy increases with an increase in heat. Heat is the transfer of energy due to temperature difference. If heat is being transferred from one object to a second object, the second object has an increase in entropy (and the first object has a decrease in entropy). The temperature for ΔS = ΔQ/T is kept constant, only entropy and heat transfer is changing. The entropy change for the hot object will be (-Q/T), with the minus sign applied because the heat is transferred away from the object. For the cold object, the entropy change is (Q/T), positive because the heat is transferred into the object. Increase in temperature results in a positive change in entropy (an increase, even if it's small). Entropy always increases with increasing temperature, the ΔS = ΔQ/T relates the change in entropy at different temperatures. |
Entropy is extensive - it increases with number, volume, and temperature.
|
|

What is free energy G?
|

The Gibbs free energy is the maximum amount of non-PV work (work not involved in expanding a system) that can be extracted from a reaction. ΔG = ΔH - TΔS. T is temperature in Kelvin.
ΔS of the SYSTEM can be <0, but ΔS of the UNIVERSE still increases regardless. So ΔG is <0 to be spontaneous, ΔG = 0 to be at equilibrium, and ΔG>0 to NOT be spontaneous. Look at the equation for Gibbs free energy, there are 2 variables for the negative sign (-TΔS), and 1 positive variable (ΔH). So Gibbs is usually negative (spontaneous). If it's not spontaneous, then it's positive. That means that it runs spontaneously in the reverse direction. ΔG = 0 means it's in equilibrium. ΔS = 0, the process is in equilibrum. ΔS<0 means the system moves to less disorder, but still ΔS of the surroundings is >0. Gas is more disordered than liquids, and liquids are more disordered than solids. Therefore, if a reaction causes a change in state from solid to liquid (melting) or liquid to gas (boiling) or solid to gas (sublimation), then the process became more disordered. Changing the other way around decreases disorder (entropy). In an isolated system such as the room and ice water taken together, the dispersal of energy from warmer to cooler always results in a net increase in entropy. Thus, when the "universe" of the room and ice water system has reached a temperature equilibrium, the entropy change from the initial state is at a maximum. The entropy of the thermodynamic system is a measure of how far the equalization has progressed. A reaction runs in a direction that increases entropy of the universe. When it has maximumized the entropy of the universe, it has reached equilibrium. |
Gibbs free energy is at a minimum when a reaction is at equilibrium. Gibbs free energy of a reaction = Gibbs free energy of the products - Gibbs free energy of the reactants. Calculating Gibbs free energy of a reaction is the same as calculating enthalpy and entropy change of a reaction. Make sure to multiply by the number of moles of the species. If you are given the product and reactant values, don't need to figure out the equation to make the reaction, just subtract them. Gibbs energy is not conserved in the conservation of energy law sense. An isolated system can change is Gibbs energy.
|
|
|
What are Spontaneous reactions and ΔGº?
|
Spontaneous reactions are reactions that can occur all by itself.
Spontaneous reactions have negative ΔG. Do not assume that an exothermic reaction is spontaneous, because a large, negative ΔS can cause it to become nonspontaneous. Do not assume that an endothermic reaction is nonspontaneous, because a large, positive ΔS can make it spontaneous. Do not assume that spontaneous reactions will occur fast, because it may take a million years for it to happen, depending on its kinetics. |
|
|
|
What is the Zeroth law (concept of temperature)?
|
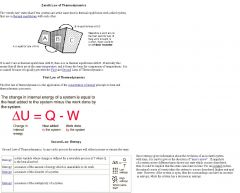
0th law of thermodynamics states "2 systems in thermal equilibrium with a third system are in thermal equilibrium with each other". The zeroth loaw declares that 2 bodies in thermal equilibrium share a thermodynamic property that is a state function. The thermodynamic property of the zeroth law is temperature.
Mathematically, if TA = TB, and TB = TC, then TA = TC. Where T is temperature. No object can reach a temperature of absolute zero. Heat flows from hot to cold - If heat is flowing from left to right through a number of slabs, then the slabs get colder from left to right. This also means that the rate of heat flow is the same for each of the slabs. The translational, rotational, and vibrational energies describes the energies of molecular motion. The sum of these energies is called thermal energy. Any increase in thermal energy increases temperature. Think of temperature as a measurement of how fast the molecules are moving or vibrating. When substances get hot, it is because their molecules move faster. For a fluid (gas and liquid), temperature is directly proportional to the translational kinetic energy of its molecules. |
Thermodynamics is the study of energy and its relationship to macroscopic properties of chemical systems. Thermodynamic functions are based on probabilities, and are valid only for systems composed of a large number of molecules. With few exceptions, the rules of thermodynamics govern complex systems containing many parts, and they cannot be applied to a specific microscopic phenomena. The average kinetic energy of a single molecule in any fluid is given by : KE = 3/2kT, where k is Boltzman's constant (k = Avogadro's number/R). For solids at high temperatures, temperature is proportional to the average kinetic energy of the vibration of molecules. Solids at lower temperature deviate from the temperature and kinetic energy proportionality because they have other effects. Solids do not translate or rotate (like fluids), they vibrate only. This is why they have greater deviation from the equation. The greater the random translational energy of gas molecules per mole, the greater the temperature. Ideally, the temperature can be thought of as the thermal energy per molecule or mole of molecules. Energy and number of moles are extensive properties. This makes temperature an intensive property. Virtually all physical properties change with temperature (thermal expansion). The greater the random translational kinetic energy of a gas per volume, the greater the pressure.
|
|

What is the First law
(ΔE = q + w, conservation of energy)? |

1st law of thermodynamics is based on the principle of conservation of energy, and it basically says that the change in total internal energy of a closed system is equal to the contributions from heat and work. This simply states that there are only 2 ways that energy can leave or enter a system: work and heat. If a system does not change its state during a process, then the ΔE=0. ΔE is never negative.
ΔE is the same thing as ΔU, which is the change in internal energy. Q is the contribution from heat Q is positive when heat is absorbed into the system (ie. heating it). Q is negative when heat leaks out of the system (ie. cooling it). W is the contribution from work. W is positive when work is done on the system (ie. compression). W is negative when work is done by the system (ie. expansion). Work is also PΔV. So if you see E = q - w, that means there is heat flow into the system and work is being done by the system. The system is losing energy because work is being done by the system. If the net work done ON a gas is a certain amount, like 55J, then the net work done BY the gas is just the negative of the net work ON the gas. We know that U is a state function and that ΔU is independent of path. However, w is not a state function so that w depends on path. Yet the sum of w and q is path independent. The only way this can happen is if q is also path dependent. We now see that we are dealing with two path-dependent quantities, q and w. |
If the system is the same temperature as its surroundings, then there can be no heat. Any energy change to such a system must be accomplished through work. Sum the change in energy and you have the work done on the system. If the system is not the same temperature as the surroundings, then heat must be considered and you have a thermodynamics problem. There are only 2 ways to transfer energy between systems: heat and work. Heat is the natural transfer of ENERGY from a warmer body to a cooler body. Any energy transfer that is not heat is work. The first law of thermodynamics states that energy of the system and surroundings is always conserved.
|
|
|
What is the equivalence of mechanical, chemical, electrical and thermal energy units?
|
If it's energy, it's Joules. It doesn't matter if it's potential energy, kinetic energy, or any energy - as long as it's energy, it has the unit Joules.
Energy is equivalent even if they are in different forms. For example, 1 Joule of mechanical energy can be converted into 1 Joule of electrical energy (ignoring heat loss) - no more, no less. |
|
|

What is the second law (concept of entropy)?
|

The 2nd law states that the things like to be in a state of higher entropy and disorder.
An isolated system will increase in entropy over time. An open system can decrease in entropy, but only at the expense of a greater increase in entropy of its surroundings. The universe as a whole is increasing in entropy. ΔS ≥ q / T q is the heat transferred. T is the temperature in Kelvin. For reversible processes ΔS = q / T. Only ideal reactions create zero universal entropy change of the universe. ΔS = 0. For irreversible processes ΔS > q / T. Real processes on a macroscopic scale are never reversible, so entropy change is always greater than the heat transfer over temperature and is always >0. Entropy is a state function. Because of the irreversibility nature of real processes, as long as anything occurs, the entropy of the universe increases. To calculate the change in entropy of a reaction, it is: (entropy of the products) - (entropy of the reactions). Entropy is an extensive property, so mulitiply the standard entropies by the number of moles. Make sure to use a balanced equation when finding the number of moles. If the question asks for the entropy of a product that has a different number of moles from the reaction, calculate the entropy using the balanced equation, then divide or multiply to get the correct product. |
The second law of thermodynamics also states that heat cannot be changed completely into work in a cyclical process. Entropy is nature's tendency to create the most probable situation that can occur within a system. The entropy of a system can decrease, only if, at the same time, the entropy of the surroundings increases by a greater or equal magnitude. This is because ΔS of the system + ΔS of the surroundings = ΔS of the universe ≥ 0. The entropy change of a forward reaction is equal to the negative entropy change of a reverse reaction (true for change of system, surroundings, universe). Thermodynamic properties are valid only for macroscopic systems. On a microscopic scale, all real chemical reactions are reversible. View entropy as nature's effort to spread energy evenly between systems. Nature likes to lower energy of a system when it is high relative to the energy of the surroundings and nature likes to rais energy of a system when it is low relative to the energy of the surroundings. It is entropy and not energy that drives reactions in a given direction. Entropy dictates whether or not a reaction will proceed. A reaction can be unfavorable in terms of enthalpy or even energy, and still proceed, but the reaction must increase the entropy of the universe to proceed. Every system seeks to achieve a minimum of free energy. This free energy is ΔG. So entropy drives the reaction because the entropy of the universe must be either 0 or positive, while the free energy seeks to be negative (at a minimum). In fact, equilibrium is the point in the reaction where the universe has achieved maximum entropy. A thermodynamically reversible reaction is one that remains infinitely close to a state of equilibrium at all time by moving infinitely slowly and making infinitely small changes to the system, each on establishing a new equilibrium. Equilibrium is established when energy is spread most evenly (forward = reverse).
|
|
|
What is the temperature scales and the conversions?
|

K = °C + 273
F = °C x 1.8 + 32 |
|
|

What is Heat transfer: conduction, convection, radiation?
|

Conduction: heat transfer by direct contact (via molecular collisions). Requires things to touch. Conduction occurs least through gas because gas molecules are farthest apart and when molecules are far apart, there are few collisions and so there is a resistance to conduction.
Convection: heat transfer by flowing current. Need fluid (gas or liquid) movements to transfer energy. This fluid flow could be air, so a lid prevents air from coming out through convection. However, non-moving air in pockets or hollow walls can also be a good insulator and can cut down on conduction because the air itself does not transfer heat well since they are not moving and is just another barrier of energy via molecular collisions (direct contact). Once air is moving, then heat can be transfered by convection. Radiation: heat transfer by electromagnetic waves (commonly in the infra-red frequency range). Does not need the physical flow of matter, can occur through a vacuum. Black box radiators absorb radiation (like absorbing light), and then emits it as radiation energy (like emitting photons). Reflected waves are simply those waves that are neither transmitted nor absorbed, but are reflected from the surface of the medium they encounter. An object that does not absorb all incident light will also emit less radiation than an ideal black body. When it's hot outside, to keep cool, people where white to reflect the light (because white clothing doesn't absorb as much radiation energy as black clothing), while in the cold, people wear black because black clothing absorbs radiation energy (then emits it as radiation energy). q = mcΔT. Don't confuse ΔT to things that change in temperature from hot to cold, ΔT can be for exothermic and endothermic reactions. For instance, if a reaction is left out in the air allowing for convection, - so an exothermic reaction that is supposed to result in the solution heating up can have a smaller ΔT (heat less) if the heat rises and leaves the solution from convection in the air. Also, an endothermic solution that is supposed to result in the solution cooling down can have a smaller ΔT (cool less) if heat from the environment enters the solution and heats it up. Calorimeters that have 2 styrofoam cups use the first cup to reduce convection (the movement of molecules within fluids - liquids and gases). Convection through a single cup equals zero because it requires some fluid to pass thru the surface. Conduction is heat (energy) transfer between molecules from region of higher temp (kinetic energy) to lower temp. The 2nd cup is an insulation barrier between what's inside the calorimeter and anything that the calorimeter touches on the outside - so that no heat is transferred from the inside to the outside. |
Heat is movement of ENERGY via conduction, convection or radiation, always from hot to cold. In conduction, higher energy molecules of one system transfer some of their energy to lower energy molecules of another system via molecular collisions. Heat can also be conducted through a single object. In convection, difference in pressure or density drive warm fluid in the direction of cooler fluid. For example, on a warm day at the BEACH, air above the land heats up faster than air above the water. As the air above the land warms, it becomes less dense than the air above the water and rises because it's lighter than the air above the water. When it rises, it carries thermal energy with it.
|
|

What is heat of fusion and heat of vaporization?
|

Also called latent heat of fusion, enthalpy of fusion and latent heat of vaporization, enthalpy of vaporization.
Heat of fusion = ΔHfus = the energy input needed to melt something from the solid to the liquid at constant temperature. Heat of vaporization = ΔHvap = the energy input needed to vaporize something from the liquid to the gas at constant temperature. Latent heats can be expressed as molar values such as J / mol. The energy it takes to melt a solid is ΔHfus x #mols of that solid. The energy it takes to vaporize a liquid is ΔHvap x #mols of that liquid. Latent heats can also be expressed as J / mass, where energy can be obtained by multiplying the latent heats by the mass of the substance. Energy is released when either a gas condenses into a liquid, or when a liquid freezes into a solid. The energy released is the same as the energy of their reverse processes. The expression latent heat refers to the amount of energy RELEASED OR ABSORBED by a chemical substance during a change of state that occurs without changing its temperature, meaning a phase transition such as the melting of ice or the boiling of water. The electrostatic potential energy is increasing as the particles are being separated from each other, but the temperature remains the same during the phase changes (vaporization, fusion, sublimation). So when you have isotherms, the net energy flow is into the sample to break bonds or out of the sample to form bonds during phase changes, and is zero at all other points. |
The enthalpy of formation of liquid water is H2 (g) + O2 (g) = H2O (l) and is ΔH = -285kJ. The enthalpy of formation of water vapor is H2 (g) + O2 (g) = H2O (g). ΔH for water vapor would be less negative (less exothermic) than ΔH for liquid water because more energy would be released to form the bonds of liquid water than to form the bonds of water vapor. Likewise, if the enthalpy of formation of ice from vapor would be more exothermic than the enthalpy of formation of liquid water.
|
|

What are reaction rates?
|
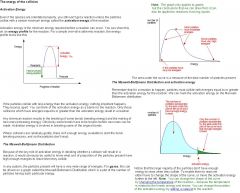
The reaction rate is defined as the rate of change in the concentration of reactants or products. ie. how fast a reactant gets used up, and how fast a product gets produced.
Rate = -ΔReactant/ΔTime = how fast a reactant disappears. Rate = ΔProduct/ΔTime = how fast a product forms. The unit for rate is molarity per second, or M/s. The reaction of a rate depends on several factors, some of which is what the rate constant depends on, like temperature, but the rate of a reaction could also depend on the surface area of the materials as they react with each other, and other things. The rate of a reaction depends on the concentration of the reactants and the concentration of the products and on catalysts. However, the rate constant does not depend on these concentrations. The rate constant ONLY depends on temperature. That's it! All reactions, whether they are endothermic or exothermic, proceed more rapidly in higher temperature. At higher temperatures, collisions occur more frequently and are more likely to have sufficient energy to initiate the reaction. Collisions must have sufficient energy and the correct spacial orientation to create a reaction - in fact, most collisions between reactants do not result in products being formed. That's why higher temperature increases the rate of a reaction because it results in a greater number of collisions to increase the change of a reaction occuring. Also realize that reaction rate increases with increasing concentration of reactants because that concentration increases the collisions that can occur with the starting molecules, increasing the chances of those molecules gaining enough energy to have a reaction. |
Kinetics deals with the rate of a reaction typically as it moves toward equilibrium, while thermodynamics deals with the balance of reactants and products after they have achieved equilibrium. Kinetics tells us how fast equilibrium is achieved, while thermodynamics tells us what equilibrium looks like.
|
|

What is the rate constant?
|

The k in the rate law is the rate constant.
The rate constant is an empirically determined value that changes with different reactions and reaction conditions. If there are more than 1 mechanisms for a reaction, there will be more than 1 rate constants (such as k1 and k2). If you see a rate equation with 2 rate constants, that means there are 2 different mechanisms for that same reaction. If you see 2 species in the rate equation for 1 rate constant, that means its a bimolecular reaction (SN2 or E2) and second order. If you only see 1 species, it's a unimolecular reaction (SN1 or E1) and first order. Both the order of the reactants and the value of the rate constant must be determined through experiment. |
Most collisions between reacting molecules do not result in a collision. The 2 requirements for a given collision to create new molecules in a reaction are 1) the relative kinetic energies of the colliding molecules must reach a threshold called the activation energy 2) the colliding molecules must have the proper spatial orientation. Chemical reactions are reversible, products begin to react to form the reactants. For rate equations, we are only considering forward reactions. Rate Constants DO NOT depend on the concentration of the reactants, rate constants, and the rate of the reaction, increase with increasing temperature. Increasing temperature of a reaction increases the energy available to BOTH the forward and reverse reactions, enabling BOTH to more easiliy overcome the activation energy. Increasing temperature increases the rate constant and rate of the reaction (both forward and reverse, just like catalysts). Just so you know, if reverse reactions are endothermic, their rate increase more (LeChatelier's Law).
|
|
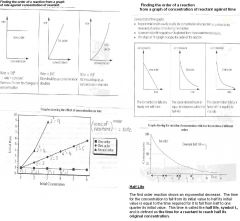
What is reaction order?
|

Reaction order = sum of all exponents of the concentration variables in the rate law.
Reaction order in A = the exponent of [A] For MCAT purposes, we will consider only reactions occurring in gases and ideally dilute liquids at constant temperature. The rate of a reaction tells us how quickly the concentration of a reactant or product is changing. Rates are most often given in terms of molarity per second (mol/(L*s)). Factors affecting the rate of a reaction are temperature, pressure, and concentration of certain substances in the reacting system, however pressure exerts on reaction rates are usually small enough to be ignored. An elementary reaction is a reaction that occurs in a single step. Multistep reactions are called complex or composite reactions. In elementary reactions, the coefficients tells you the molecularity: how many molecules participate in a reaction producing collisions. There are 3 possible molecularities: unimolecular, bimolecular, and termolecular. The MCAT will let you know what is elementary. |
Unimolecular reaction: an elementary reaction in which the rearrangement of a single molecule produces one or more molecules of product. Elementary reaction: a reaction that cannot be broken down into smaller steps. Bimolecular reaction: the collision and combination of two reactants to give an activated complex in an elementary reaction. Termolecular reaction: an elementary reaction involving the simultaneous collision of any combination of three molecules, ions, or atoms.
|
|

What is the rate-determining step?
|

The slowest step of a multi-step reaction is the rate determining step.
The rate of the whole reaction = ther rate of the rate determining step. The rate law corresponds to the components of the rate determining step. Whenever the fast step proceeds the slow step, the fast step is assumed to reach equilibrium and the equilibrium concentrations are used for the rate law of the slow step. In general, whenever a fast step proceeds a slow step, we can solve for the concentration of an intermediate by assuming that an equilibrium is reached in the fast step. |
|
|
|
What is the Activated complex or transition state of the activation energy?
|
Activated complex = what's present at the transition state.
In the transition state, bonds that are going to form are just beginning to form, and bonds that are going to break are just beginning to break. The transition state is the peak of the energy profile. The transition state can go either way, back to the reactants, or forward to form the products. You can't isolate the transition state. Don't confuse the transition state with a reaction intermediate, which is one that you can isolate. |
|
|

How do you interprete of energy profiles showing energies of reactants and products, activation energy, ΔH for the reaction.
|
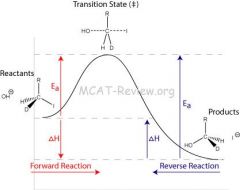
The activation energy is the energy it takes to push the reactants up to the transition state.
ΔH is the difference between the reactant H and the product H (net change in H for the reaction). H is heat of enthalpy. Exothermic reaction = negative ΔH Endothermic reaction = positive ΔH The chemical energy in a substance is a type of potential energy stored within the substance. This stored chemical potential energy is the heat content or enthalpy of the substance. You can predict what molecules will have a higher or lower amount of energy release (heat release) based on their potential energies. For instance, to determine what alkanes will have the lowest heat of combustion per CH2 group, look at their potential energies. The potential energy of a cycloalkane is a function of its torsional, steric, and ring strains. In order to make comparisons between cycloalkanes with different numbers of CH2 units, divide the heats of combusion by the number of CH2 units for each cycloalkane to get the potential energy per CH2 (because enthalpy, heat of combustion, is a measure of potential energy). Cyclohexane, when compared to cyclobutane and cyclopropane, has a minimum potential energy because it can adopt a conformation (the chair) that minimizes torsional, steric, and ring strains. When it undergoes combustion, cyclohexane yields less heat per CH2 than the other cycloalkanes because it is initially at a lower energy potential per CH2. |
The activation energy is the energy required for a collision of properly oriented molecules to produce a reaction. Activation energy does not change with temperature.
|
|
|
What is the difference between kinetic control and thermodynamic control of a reaction?
|
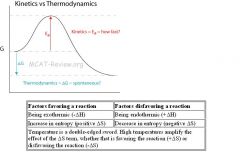
•Thermodynamics tells you whether a reaction will occur. In other words, whether it is spontaneous or not.
◦A reaction will occur if ΔG is negative. ◦ΔG = ΔH - TΔS •Kinetics tells you how fast a reaction will occur. ◦A reaction will occur faster if it has a lower activation energy. |
|
|

What are catalysts?
|

•Catalysts speed up a reaction without getting itself used up. If catalysts are consumed an a reaction, the are subsequently regenerated at the end of a reaction.
•Enzymes are biological catalysts. •Catalysts/enzymes act by lowering the activation energy, which speeds up both the forward and the reverse reaction. •Catalysts/enzymes alter kinetics, not thermodynamics. •Catalysts/enzymes help a system to achieve its equilibrium faster, but does not alter the position of the equilibrium. •Catalysts/enzymes increase k (rate constant, kinetics), but does not alter Keq (thermodynamics, equilibrium). In chemical kinetics, a reaction rate constant k or quantifies the speed of a chemical reaction. The rate constant is only constant if all you are changing is the concentration of the reactants. If you change the temperature or the catalyst, for example, the rate constant changes. Increase in speed through catalyst or increase in temperature means that k (rate constant) will increase. Catalysts work by changing the mechanism of the reaction involving a different transition state and lower activation energy. If you have two reaction chambers with the same reaction and a catalyst is used in 1 of them, the chamber with the catalyst will create the product faster, but eventually, both chambers will have the same amount of products because the catalyst does not change the proportion of products to reactants. Catalysts may also increase the steric factor (p from the Arrhenius equation), but most lower the activation energy. |
REMEMBER: Catalysts only affect the RATE of the reaction and not the EQUILIBRIUM. Catalysts also do not affect the change in enthalpy.
|
|

What is the difference between the reaction quotient, Q, and Keq?
|

◦The reaction quotient, Q, is the same as Keq except Q can be used for any point in the reaction, not just at the equilibrium.
■If Q < Keq, then the reaction is at a point where it is still moving to the right in order to reach equilibrium. ■If Q = Keq, the reaction is at equilibrium. ■If Q > Keq, then the reaction is too far right, and is moving back left in order to reach equilibrium. ◦The reaction naturally seeks to reach its equilibrium So, if a problem gives you the Keq and then, it also gives you a set of concentrations of the reactants and products, calculate the reaction quotient and compare the reaction quotient to the Keq to see if the reaction will go to the left or the right. The way to identify these questions is if they ask "What rates will the forward or reverse reaction be?" because they will give you the concentrations. We are trying to see if from those concentrations, there are too much product or too much reactant and we know this from comparing Q with K. Gases can be written either as concentrations or as partial pressures in equilibrium expressions. The two can be related by the ideal gas law, and thus their numerical values will differ, resulting in a different K for both. The chemical equation will let you know how quickly the concentration of the reactants will disappear and how quickly the products will appear based on the moles of the species - a product with a higher number of moles will increase by that molar amount and a reactant with a higher number of moles will decrease by tha molar amount - this can be exemplified in the slope of a concentration versus time graph. |
The reaction quotient is not a constant, it's a means of comparing the direction in which a reaction will proceed. If Q > K, the reverse reaction rate will be greater than the forward rate. Of course, K does not change. When Q < K, the forward reaction rate will be greater than the reverse rate. The reaction rate is separate from the rate constant, the rate constant only increases with TEMPERATURE!
|
|
|
How can you apply LeChatelier's principle to equilibrium in reversible chemical reactions?
|

◦LeChatelier's principle: If you have a system in equilibrium and you disturb it, the system responds in a way that opposes the disturbance. It undoes the disturbance. If you add heat, it takes away the heat. If you have more moles of gas on the product side, then if you increase the pressure, the reaction will move to the reactants side.
◦A reaction at equilibrium doesn't move forward or backward, but the application of LeChatlier's principle means that you can disrupt a reaction at equilibrium so that it will proceed forward or backward in order to restore the equilibrium. Remember that adding more solids or pure liquids will not affect the equilibrium because they are at constant concentrations are represented as 1 in the equilibrium expression. If a reaction is at equilibrium, temperature will also knock that reaction off balance, until the reaction returns to equilibrium - so if the reaction is exothermic and is at equilibrium, raising the temperature will cause the reaction to move to the left and lowering the temperature will move the reaction to the right. However, if the reaction hasn't yet reached equilibrium, then raising the temperature will increase the rate of the reaction in general, regardless if it's a exothermic or endothermic reaction - reaction rates always increase with increase in temperature - this is satisfied with the rate law. So temperature affects the rate and it also affects the equilibrium. |
Chemical equilibrium is when the forward reaction rate equals the reverse reaction rate. At chemical equilibrium, there is no change in the concentration of the products or reactants. Equilibrium will be reached from either direction, beginning with predominantly reactants or predominantly products. Equilibrium is the point of greatest entropy. A reaction could run to completion when the rate constant for the forward reaction is so much greater than the rate constant for the reverse reaction, or if the product is continually removed as the reaction proceeds (perhaps like gas leaving an aqueous solution), the reaction can run to completion. With LeChatelier's principle, if one side of a reaction contains more moles than the other, the equilibrium shifts to the side with fewer moles. This works with concentration or dilution and with gas molecules.
|
|

What is the relationship of the equilibrium constant and ΔGº (standard free energy change)?
|

•ΔG = ΔG° + RT ln Q
◦Set ΔG = 0 at equilibrium. ◦Q becomes K at equilibrium. •0 = ΔG° + RT ln (K) •ΔG° = -RT ln (K) K is the equilibrium constant and equilibrium constant K changes when the temperature is altered based on this equation. The rate constant k also changes with temperature, based on Arrhenius's equation. Both only change according to temperature, nothing else (not reactants, etc). The standard Gibbs free energy of formation of a compound is the change of Gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements, at their standard states (the most stable form of the element at 25° C and 100 kilopascals). Its symbol is ΔGº. Gibbs free energy change of a reaction is the maximum energy that the reaction will release to do non-P-V work only, meaning work that is not involved in expanding a system. This is useful for things like batteries and living cells where there are no expanding gases. Gibbs free energy of the universe is decreased with each reaction, so it does not follow the conservation of energy law. Nonspontaneous reactions can be caused to occur by coupling them to a more spontaneous reaction, so that the total free energy change is negative. ΔG° = ΔH° - TΔS°. You can use this equation for find the standard free energy change, the standard enthalpy change, and the standard entropy of a reaction. Also, entropy is the negative slope of a G versus T graph. |
ΔG = ΔH - TΔS applies to the SYSTEM, not the surroundings. It applies to constant temperature and constant pressure. Equilibrium is achieved when ΔG = 0 (constant T and P, PV work only, and a reversible process). A reaction where ΔG is negative indicates an increase in ΔS of the universe, and such a reaction is said to occur spontaneously. The true definition of a spontaneous reaction requires only that ΔS of the universe be positive under ANY conditions. For the MCAT, a negative ΔG is good enough for spontaneity.
|
|
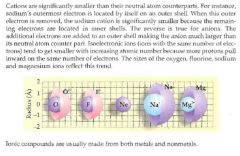
What are the common names, formulas and charges for familiar anions and cations; e.g., NH4+, ammonium; PO43-, phosphate; SO42-, sulfate?
|

|
Cations are significantly smaller than their neutral atom counterparts. For instance, sodium's outermost electron is located by itself on an outer shell. When this outer electron is removed, the sodium cation is significantly smaller because the remaining electrons are located on inner shells. The reverse is true for anions. The additional electrons are added to an outer shell making the anion much larger than its neutral atom counterpart. Isoelectronic ions (ions with the same number of electrons) tend to get SMALLER with INCREASING atomic number because more protons pull inward on the same number of electrons.
|
|

What is hydration and the hydronium ion?
|
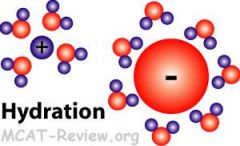
◦Another name for solvation is hydration.
◦Hydration is where water forms a shell around ions that are dissolved ionic compounds in solution. When several water molecules attach to one side of an ionic compound, they are able to overcome the strong ionic bond and break apart the compound. ◦The oxygen atom on water is partially negative, so it surrounds cations. ◦The hydrogen atoms on water is partially positive, so they surround anions. ◦Hydronium ion = H3O+ ◦H+ never exist as a proton in water, it always exists as the hydronium ion. The heat of hydration is the enthalpy change when a solute dissolves in water. It involves the breaking of water-water bonds, which is endothermic, and the forming of water-solute bonds, which is exothermic. The net result can either be endo- or exothermic and the heat of hydration can either be positive or negative. It depends on the solute. The formation of a solution involves the interaction of solute with solvent molecules. Many different liquids can be used as solvents for liquid solutions, and water is the most commonly used solvent. When water is used as the solvent, the dissolving process is called hydration. Since the heat of hydration is negative, the energy released by the formation of water-solute bonds must have a greater magnitude than the energy absorbed by the breaking of water-water bonds. Their bond energy is a measure of their strength. The water-water bonds are the hydrogen bonds between water. |
Supersaturated solutions occur when a solution is beyond it's saturation point (which is found at a certain concentration, like 37.7 g of lead/100ml of water at STP). If you equilibrate 39 g of lead in 100ml of water over a period of days at STP, it will produce an unstable, but saturated solution. In this case, it is supersaturated. Any sudden disturbances will initiate precipitation of the extra lead. Ionic compounds are dissolved by polar substances. When ionic compounds dissolve, they break apart into their respective cations and anions and are surrounded by the oppositely charged ends of the polar solvent. This process is solvation. Water is a good solvent for ionic substances. Something that is hydrated is in the aqueous phase. The number of water molecules needed to surround an ion varies according to the size and charge of the ion. This number is called the hydration number, which is commonly 4 or 6.
|
|

What are the units of concentration for solubility?
|
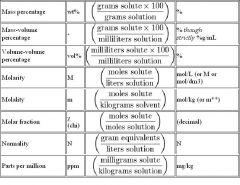
If given a solution of 3 different ions, if the concentrations are the same, the least soluble ion will precipitate first. But, we can only compare their Ksp's if they break up into equal number of particles (like NaCl and KCl). If they do break up into different numbers of particles, like CaCO3 and Na2CO3, then we can't just compare their Ksp values.
Solubility values are given in units of concentration: molar solubilities (mol/L). So if you need to figure out the solubility product (solubility product constant) for a substance, you can figure it out by creating an equation for the Ksp and using the solubility concentrations of the products (i.e. Ksp = [A][B], and we know the solubility concentrations of A and B, so we just multiply them to get the Ksp). The Ksp is computed from molar solubilities. That is why we can figure out the pH of substances! All [H+] and [OH-] is is solubility concentrations of H+ and OH- of the acids and bases. So if we are given a molar value, such as 0.01 M NaOH, we can figure out the pH of this because we know that there is a concentration of 0.01 moles/L of OH-. We just plug that into the pOH = -log[OH-] equation and get pOH! It's that easy! If a molecule is really stable and has strong intermolecular bonds, it has a lower solubility than other molecules that are less stable with less strong intermolecular bonds. |
If asked to find the mole fraction (X) of a solute in a 10% (wt-wt) aqueous solution, assume that there is 100g of solution (solute + solvent). Then 10% will the solute (10g) and 90% will be the solvent (90g). Convert the grams to moles to determine the mole fraction (moles of solute /(moles of solute + moles of solvent). On the MCAT, the solute will usually be a salt and the solvent will most often be water.
|
|
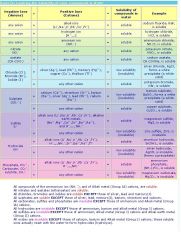
What is the solubility product constant in the equilibrium expression?
|

Solubility product constant is simplified equilibrium constant (Ksp) defined for equilibrium between a solid and its respective ions in a solution. Like any equilibrium constant, it is unitless. Its value indicates the degree to which a compound dissociates in water. The higher the solubility product constant, the more soluble the compound. The solubility product does not change with changing ion concentration, it only changes with temperature. The Ksp expression for a salt is the product of the concentrations of the ions, with each concentration raised to a power equal to the coefficient of that ion in the balanced equation for the solubility equilibrium. Solubility product constants are used to describe saturated solutions of ionic compounds of relatively low solubility. A saturated solution is in a state of dynamic equilibrium between the dissolved, dissociated, ionic compound and the undissolved solid. Saturation is the point at which a solution of a substance can dissolve no more of that substance and additional amounts of it will appear as a precipitate. Nitrates and sodium salts are soluble in water. Metals and nonmetals which form salts like potassium oxide are strong electrolytes and are ionic. The solubility product constant is an equilibrium constant expression. It does not describe shifts in equilibrium like Le Chatlier's principle - that describes the SOLUBILITY of something, that is a qualitative view of solubility. An unsaturated solution is a solution that can dissolve more solute at a given temperature. To determine if something is saturated or unsaturated, find the Q constant of the equation, using the concentrations, and compare to the Ksp. If Q<K, the solution is unsaturated. If Q=K, the solution is saturated at equilibrium). For an ionic compound whose formula unit contains one cation unit and one anion unit of an equal and opposite charge, the solubility S (or molar solubility) is equal to the square root of Ksp - because Ksp is equal to the concentration of both the ionic compounds, in equal proportions.
|
With chemical equations, you can find the concentration of the reactants to the reactants and the products to the products by setting up a ratio. Say you have 1O + 2H yields 3C + 4N. The concentration of O with respect to H is 1/2[H] and the concentration of C with respect to N is 3/4[N]. Saturation is when the rate of dissolution and precipitation are equal. For most salts, crystallization is an exothermic reaction. When a solution is saturated, the concentration of the solute is at a maximum and the concentration of the solvent is at a minimum.
|
|

What is the solubility of MX2 if given Ksp?
|
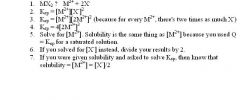
Solubility and the solubility product are NOT the same thing. Solubility product (solubility product constant) or Ksp is a constant found in a book. Solubility is the maximum number of moles of solute that can dissolve in a liter of solution. Solubility can be found using molarity (M). Solubility depends on the common ions in the solution whil the solubility constant is independent of the common ions. The solubility product changes only with temperature (like the rate constant). The solubility depends upon the temperature and the ions in the solution (analogous to the rate of an equation). When solving solubility equations, we call the solubtility 'x' since it is unknown, and x is the moles per liter of the compound. Pressure and temperature affect solubilities. Pressure on liquids and solids has little effect (because they are incompressible), but pressure on a gas increases its solubility (because gases are compressible). So increased pressure and decreased temperature increases the solubility of a gas in a solution.
|
|
|

What is the Common-ion effect and its use in laboratory separations?
|

•The common-ion effect is simply Le Chatelier's principle applied to Ksp reactions. The common-ion effect is the lowering of the degree of ionization of a compound when another ionizable compound is added to a solution; the compound added has a common ion with the other compound.
•AgCl (s) ↔ Ag+ (aq) + Cl- (aq) •The common-ion effect says that if you add Cl- to the solution above, then less AgCl would dissolve. •For example, if you add NaCl to a saturated solution of AgCl, then some AgCl will crash out of solution. •Another example: more AgCl can dissolve in pure water than in water containing Cl- ions. •In laboratory separations, you can use the common ion effect to selectively crashing out one component in a mixture. ◦For example, if you want to separate AgCl from a mixture of AgCl and Ag2SO4, then you can do so by adding NaCl. This will selectively crash out AgCl by the common ion effect (Cl- being the common ion). Ionic compounds normally dissociate into their constituent ions when they dissolve in water. When writing the equations, you do not have to include H2O in the expression. The common ion effect occurs to allow the solubility equilibrium to shift until the concentration of the ions in solution are such that they again satisfy the solubility product. |
|
|
|
What is Complex ion formation?
|
•The "complex ion effect" is the opposite of the common ion effect. Complex ions are formed when an anion (Lewis base) is reacted with a metal cation (Lewis acid). Of all the elements, the transition metal ions tend to create complex ions. This tendency is due to the fact that transition metals have at least one oxidation state.
•AgCl (s) ↔ Ag+ (aq) + Cl- (aq); M+ + Cl- ↔ M-Cln complex ion. •When complex ion forms, the Cl- ion is taken out, so more of the AgCl will dissolve. •Alternatively: AgCl (s) ↔ Ag+ (aq) + Cl- (aq); NH3 + Ag+ ↔ Ag-(NH3)n complex ion. •Here, the complex ion formation takes out Ag+, again causing more AgCl to dissolve. |
|
|

Explain the relationship between solubility and pH?
|

•Acids are more soluble in bases.
◦HA → H+ + A- ◦Putting the above in a base (OH-) will take out the H+, thus, more HA will dissolve according to Le Chatelier's principle. •Bases are more soluble in acids. ◦B + H+ → BH+ ◦Putting the above in an acid will add more H+, and thus, drive more B to dissolve according to Le Chatelier's principle. The extent to which an electrolyte dissolves in solution is not related to whether or not it is soluble. Acetic acid is a weak electrolyte which is very soluble in water. The difference is, electrolytes are strong or weak based on how they IONIZE in solution (fully or partially), but the fact that they are soluble at all in solution is a different story. |
Le Chatalier's principle when applied to solutions should be used with caution. Remember, salts increase in solubility in solution when temperature is increased, even with a negative heat of solution. Solution formation always increases in entropy, and this increase is so large that it doesn't matter if the entropy change is negative. The increase in T of the Gibbs energy equation makes Gibbs free energy more negative.
|
|
|
What is the Brønsted–Lowry definition of acids and bases?
|
◦H-Acid + Base- ↔ Acid- + H-Base.
◦From left to right: ■Acid: proton donor. ■Base: proton acceptor. ■Conjugate base: acid after losing its proton. ■Conjugate acid: base after gaining its proton. |
|
|

What is Kw (solubility product constant of water), its approximate value in relation to the ionization of water?
|
Kw = [H+][OH-] = 1*10^-14 at 25° C.
■H2O ↔ H+ + OH- ■At standard conditions, pure water dissociates to achieve [H+] = 10^-7 M and [OH-] = 10^-7 M. ■Kw = [H+] x [OH-] = 10^-7 x 10^-7 = 10^-14 Not all acids completely ionize. For instance, if you have an acid like H2SO3, and you are given a really small Ka (dissociation constant), for the ionization of HSO3 to SO3, then it is ok to assume that the acid behaves like a monoprotic acid (H2SO3 to HSO3). So, if you needed to figure out the pH, you would only figure it out as if it was a monoprotic acid, not a diprotic acid. Pure water contains some H3O+ as a result of autoionization - it ionizes with itself: H2O(l) + H2O(l) yields H3O+(aq) + OH-(aq) |
|
|

What is the pH definition and the pH of pure water?
|
■pH = -log[H+]
[H+] the brackets always indicate concentration, like moles/liter ■For pure water, pH = -log[10^-7] = 7. ■Acidic: pH lower than 7. ■Neutral: pH = 7. ■Basic: pH higher than 7. ■pOH = -log[OH-]. ■pH + pOH = 14. The pH value is a measure of the acidity or basicity of a solution. Pure water is neutral, and can be considered either a very weak acid or a very weak base (center on the pH scale), giving it a pH of 7. Water is amphoteric in that it acts as a base (by accepting a proton) or acid (by donating a proton) - it undergoes auto-ionization. |
Each point on the pH scale corresponds to a tenfold difference in hydrogen ion concentration. An acid with a pH of 2 produces 10 times as many hydrogen ions as an acid with a pH of 3, and 100 times as many hydrogen ions as an acid with a pH of 4.
|
|
|
What are Conjugate acids and bases (e.g., amino acids)?
|

Within the Brønsted-Lowry (protonic) theory of acids and bases, a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical reaction, hence, the term acid. The base produced, X−, is called the conjugate base, and it absorbs, or gains, a proton in the backward chemical reaction. In aqueous solution, the chemical reaction involved is of the form
HX + H2O X− + H3O+ The stronger an acid, the weaker its conjugate base, and, conversely, the stronger a base, the weaker its conjugate acid. The strong acid loses the proton easily leaving behind a weak base. And strong bases gains the proton easily, leaving behind a weak acid. However, this does NOT mean "Strong acids have weak conjugate bases, and weak acids have strong conjugate bases". Weak acids do not necessarily have strong conjugate bases. A weak acid may have a strong or weak conjugate base. So we only look at it with respect to STRONG ACIDS and STRONG BASES - The STRONGER the acid, the weaker its conjugate base, and the STRONGER the base, the weaker its conjugate acid. Acid strength is on the logarithmic scale. |
|
|
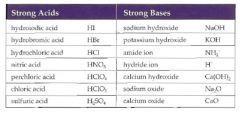
What are Strong acids and bases (common examples; e.g., nitric, sulfuric)?
|

◦Strong acids completely dissociate in solution.
◦Complete dissociation occurs because the conjugate base anion is highly stable. ◦Strong bases completely dissociate in solution. ◦Complete dissociation occurs because the conjugate acid cation is highly stable. Strong acids and strong bases dissociate completely in solution, but different dissociation concentrations exist when there is limited solution. For instance, H2SO4 ionizes completely in water because it is a strong acid. However, if there is very little water content, all of the H2SO4 can't ionize. So the first ionization of H2SO4 in water will yield HSO4- and H3O+. There would need to be more water available for the HSO4- to undergo a second ionization of to yield SO4^2- and H3O+. So SO4^2- would be the least amount in the concentration compared to HSO4-, and HSO4- would be smaller in concentration than H2SO4. So when there is limited water to dissociate acids and bases, pay attention to how much the acid and base needs to be ionized in order to see how much of the dissociation species would be in the solution. |
When we say "Strong acid", we mean an acid that is stronger than H3O+ (H+). A strong base is stronger than OH-. Which bases, we often call something as strong as OH-, like NaOH, a strong base. For MCAT purposes, we assume that a strong acid or base COMPLETELY dissociates in water.
|
|
|
What are Weak acids and bases (common examples; e.g., acetic, benzoic)?
|

◦Weak acids partially dissociate in solution.
◦Partial dissociation occurs because the conjugate base is fairly stable. ◦Weak bases partially dissociate in solution. ◦Partial dissociation occurs because the conjugate acid is fairly stable. |
|
|
|
Explain the dissociation of weak acids and bases with or without added salt.
|
■CH3COOH will dissociate less in a solution containing CH3COONa salt (which is the salt of the weak acid CH3COOH).
■NH4OH will dissociate less in a solution containing NH4Cl salt (salt of weak base NH4OH). ■This is due to Le Chatelier's principle: the dissociation of salts like CH3COONa of weak acids will produce the their conjugate bases (CH3COO-), which reduces dissociation because the weak acid CH3COOH will also dissociate into CH3COO- and H+ and this extra CH3COO- will drive the reaction to the left. Likewise, dissociation of salts like NH4Cl of weak bases will produce conjugate acids like NH4. The dissociation of NH4Cl will produce NH4+ and Cl-. This will drive the reactions to the left because NH4OH will also dissociate into NH4+ and OH- and the extra NH4+ drives the reaction to the left and lowers dissociation. Without the salt, weak acids and weak bases only partially dissociate in solution, whereas strong acids and strong bases completely dissociate in solution. The conjugate acids of weak bases are weak acids and conjugate bases of weak acids are weak bases. |
|
|

What are the Equilibrium constants Ka and Kb (pKa and pKb)?
|
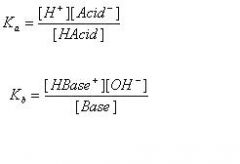
◦H-Acid ↔ H+ + Acid-
◦Base + H2O yields H-Base+ + OH- note: water is not included in the formula because it is not a solute. ◦Ka x Kb = Kw = 10^-14 ◦pKa = -log Ka ◦pKb = -log Kb ◦pKa + pKb = 14 The higher the Ka, the stronger the acid. The higher the Kb, the stronger the base. The smaller the pKa, the stronger the acid, the smaller the pKb, the stronger the base. Ka is acid ionization constant or the equilibrium constant for the ionization of a weak acid in water to produce hydronium ions and the conjugate base of the acid. Kb is base ionization constant or the equilibrium constant for the ionization of a weak base in water to produce hydroxide and the conjugate acid of the base. |
The concentration of the conjugate base to the acid is related. [A-]/[HA] = 10^(pH-pKa).
Ka and Kb are acid and base equilibrium constants. They are also presented as pKa and pKb, and pKa and pKb are also referred to as acid dissociation constants. pKa = -logKa |
|
|
What is the definition of buffers and the concept of common buffer systems?
|

■Buffers = Solutions that resist changes in pH.
■Salts of weak acids and bases form buffer systems. ■A buffer system consists of an equilibrium between an acidic species and a basic species. Note the "equilibrium", you can't just dump HCl and NaOH together and expect buffering, because neutralization will occur and the acidic species and the basic species won't be at an equilibrium. ■The concept is that acidic species of the buffer system will donate protons to resist increases in pH, while the basic species of the buffer system will accept protons to resist decreases in pH. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value |
A buffered solution is formed when equal amounts of a weak acid and its conjugate base are present in a solution. For instance acetic acid HC2H3O2 is a weak acid and the acetate ion NaC2H3O2 is its conjugate in a buffer solution (Na is a spectator ion).
|
|
|
What is the influence of buffers on titration curves?
|
■Buffers make the titration curve "flat" at the region where buffering occurs. On a titration curve, this is called the point of inflection (a point on a curve at which the curvature changes signs).
■The point of inflection is at pH = pKa of the buffer and this is the buffering capacity. It is the maximum amount of either strong acid or strong base that can be added before a significant change in the pH will occur. A buffer solution is at its maximum buffering capacity when the ratio of base to conjugate acid or acid to conjugate base is 1. ■The area around the point of inflection is the region where the solution has buffering capacity. The pH of this buffering region is typically pKa +/- 1. This is the approximate range within which buffering by a weak acid is effective. |
|
|
|
What are the indicators of titration?
|

◦H-In ↔ H+ + In-
◦Ka = [H+][In-] / [H-In] ◦Indicators behave just like weak acids/bases. ◦The indicator is present is such a small amount that it doesn't affect the solution's pH. ◦When the solution has a low pH (high [H+]), the indicator is mostly in the H-In form, which is of one color. ◦When the solution has a high pH (low [H+]), the indicator is mostly in the In- form, which is of another color. The point where the indicator changes color is called the endpoint (think Endicator). |
An indicator generally changes color within plus or minus 1 pH point of its pKa.
|
|
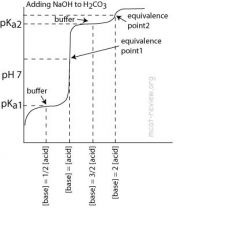
How do you interprete titration curves?
|
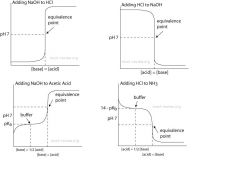
■At the point of inflection (buffer arrow), the [acid analyte] = [conjugate base analyte] or [base analyte] = [conjugate acid analyte], pH = pKa, and [titrant] = 1/2 [weak acid/base analyte]
■The buffer region has pH values of pKa +/- 1. ■Polyprotic acids have multiple pKa, points of inflection, and equivalence points. ■Like monoprotic acids, each point of inflection corresponds to the pKa for the acidic species. ■At each pKa, [acidic analyte species] = [conjugate base of the acidic analyte species]. ■For H2CO3 analyte: ■pKa1: [H2CO3] = [HCO3-] ■Equivalence point 1: the [titrant] is stochiometrically equal to the [analyte] - meaning the titrant and analyte are mixed together in exactly the proportions of their balanced equations. The equivalence point is the steepest part of the curve. ■pKa2: [HCO3-] = [CO32-] ■Equivalence point 2: the [titrant] is stochiometrically equal to the [analyte] - meaning the titrant and analyte are mixed together in exactly the proportions of their balanced equations. a diprotic acid, which means that it can give away 2 protons (hydrogen ions) to a base. Something which can only give away one (like HCl) is known as a monoprotic acid. Diprotic (and higher) acids have multiple equivalence points and inflection points. |
For diprotic acids, the second proton donated by the polyprotic acid is usually so weak that its effect on the pH is negligible. On the MCAT, the second protocol can almost always be ignored. The rule of thumb is that if the Ka values differ by more than 10^3, the second proton can be ignored.
An equivalence is the mass of acid or base necessary to produce or consume one mole of protons. The equivalence point is not necessarily when the analyte and titrant are at equal VOLUMES. If the concentrations differ, and they probably will, the equivalence point will not be where the volumes are equal. The point of inflection is also called the half equivalence point. |
|
|
What is electrolysis?
|
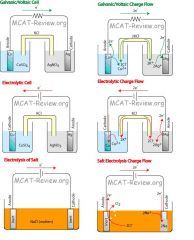
Electrolysis is a method of using an electric current to drive an otherwise non-spontaneous chemical reaction.
◦Requires potential/voltage input. On the diagram, this is represented by a battery in the circuit. In contrast, a galvanic cell has in its place either a resister, or a Voltmeter. ◦ In general, the potential/voltage input + the cell potential must be > 0 for reactions to occur. ◦For electrolytic cells, the cell potential is negative, so a potential input greater than the magnitude of the cell potential must be present for electrolysis to occur. ◦In contrast, galvanic/voltaic cells already have a positive cell potential. Thus, no input is required for galvanic/voltaic cells. ◦In the diagram above, arrows are shown in red because the battery is forcing the flow of electrons. Normally, the electrons would want to flow the other way (or not flow at all). |
|
|
|
What are anodes and cathodes?
|

◦The following rules hold true for both electrolytic and galvanic/voltaic cells.
◦Anode is always the place where oxidation happens. ◦Cathode is always the place where reduction happens. ◦Mnemonic: ■An Ox = ANode OXidation ■Red Cat = REDuction CAThode ◦Anode shoots out electrons, Cathode takes in electrons. When calculating E values, remember - DO NOT MULTIPLY OR DIVIDE THE E VALUES! E values are intensive properties and do not depend on the size of the system (as opposed to enthalpy, which does depend on the size and which we do multiply based on balanced coefficients). Just add (or subtract) E values. E values are subtracted when you are using the reverse reaction. BUT DON'T MULTIPLY OR DIVIDE! |
|
|

What are electrolytes?
|
◦Ions = electrolyte.
◦Electrolytes conduct electricity by the motion of ions. ◦Without electrolytes, there won't be a circuit because electricity won't be able to travel. When ions form in aqueous solution, the solution is able to conduct electricity. A compound which forms ions in aqueous solution is called an electrolyte. Strong electrolytes create solutions which conduct electricity well and contain many ions. Weak electrolytes are compounds which form few ions in solution. |
|
|
|
What is electron flow and oxidation and reduction at the electrodes?
|
◦Electrons shoot out of the anode because oxidation occurs there to lose electrons. M → M+ + e-.
◦Electrons travel into the cathode, where it crashes into the cations on the surface of the cathode. This is because reduction occurs at the cathode to receive electrons. M+ + e- → M. ◦Mnemonic: ■Oil Rig : Oxidation Is Losing e- Reduction Is Gaining e-. ■Oxidation is an increase in charge, Reduction is a decrease in charge. |
Also remember: RED CAT; AN OX: reduction cathode, anode oxidation.
Since we are working with electrons, they have a negative sign. The flow of electrons is from the anode to the cathode, so the anode repels the electrons by having a negative sign and the cathode attracts the electrons by having a positive sign. |
|
|
What are half-reactions?
|
◦Oxidation half reaction describes the species that loses electrons (increases in charge). For example, Cu → Cu2+ + 2e-
◦Reduction half reaction decribes the species that gains electrons (decreases in charge). For example, 2Ag+ + 2e- → 2Ag |
|
|
|
What are reduction potentials?
|
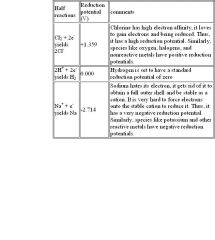
◦Reduction potential = potential of the reduction half reaction.
◦Oxidation potential = potential of the oxidation half reaction = reverse the sign of the reduction potential. Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts (V) and the more positive the potential, the greater the species' affinity for electrons and tendency to be reduced. Oxidation potential measures the tendency for a chemical species to lose electrons and become oxidized. The more negative the reduction potential, the greater the species's tendency to be oxidized. The reduced species of the electrochemical equilibrium with the most negative E value is the strongest reducing agent. The reducing agent reduces the other substance, so it is oxidized. The more negative the E value, the more oxidized the substance is. The strongest reducing agent will have the most negative E value, because the negative E value tells you the oxidation potential. The reduction potential is positive. If you have a reaction where a species is oxidized, but has a negative E value, that tells you that the cell is not spontaneous. Only reactions with species that have a positive E value are spontaneous. It could have an oxidation and a reduction occur, but the overall potential has to have a positive E value. |
When a question asks about how many electrons are being transferred, evaluate the oxidation/reduction of the species and see the number of electrons that are transferred between the oxidized species and the reduced species.
|
|
What are cell potentials for galvanic cells?
|

◦Cell potential = Reduction potential + Oxidation potential.
the cell potential for the galvanic cell shown in the diagram is shown. ■Reduction half reaction: 2Ag+ + 2e- → 2Ag ■Reduction potential = +0.799 ■Oxidation half reaction: Cu → Cu2+ + 2e- ■Oxidation potential = +0.337 x -1 = -0.337 ■Cell potential = 0.799 - 0.337 = 0.462 V ■The cell potential for all galvanic/voltaic cells is positive, because the voltaic cell generates potential. |
If you need to look at the oxidation potential of a reaction, and you are using a reduction potential table, remember that not only do you have to reverse the sign of the reduction potentials, you also have to REVERSE the half REACTION.
|
|
What is the the cell potential for the electrolytic cell shown in the diagram?
|
■Reduction half reaction: Cu2+ + 2e- → Cu
■Reduction potential = +0.337 ■Oxidation half reaction: 2Ag → 2Ag+ + 2e- ■Oxidation potential = +0.799 x -1 ■Cell potential = 0.337 - 0.799 = -0.462 V ■The cell potential for all electrolytic cells is negative, because the electrolytic cell requires potential input. |
For electrolytic cells, reduction still takes place at the cathode and oxidation at the anode. It's just that the species getting oxidized naturally want to be reduced, and the species getting reduced naturally want to be oxidized, so the reaction requires energy. Electrons always flow from anode to cathode, regardless of the cell.
|
|
|
What is the direction of electron flow for galvanic (voltaic) cells?
|
◦Electrons always flow from the Anode to the Cathode. Mnemonic: A to C in alphabetical order. Or, think about AC power - the A comes first and stands for anode)
◦Oxidation (at the anode) produces electrons (and cations), and shoots out the electrons toward the cathode. The cathode receives those electrons and use them for reduction. ◦Naturally, the species with the highest oxidation potential (lowest reduction potential) will be the anode, and the species with the highest reduction potential will be the cathode. ■In the diagram above, the Galvanic/Voltaic cell shows a natural flow because Cu (higher oxidation potential/lower reduction potential) is the anode, and Ag (higher reduction potential) is the cathode. ■However, the electrolytic cell shows exactly the opposite. In order to force the Cu to be the cathode and Ag to be the anode, a battery is used to drive the reaction. ◦Electrons flow in wires and electrodes, while ions flow in the electrolyte solution, thus creating a completed circuit. |
|
|

How do shell stability mess with electron configuration?
|
Electronic configurations that change because of filled/half-filled shells are all the d4 and d9 electron configurations. In each case, a single electron in the s2 orbital is
relocated to the d5 or d10 orbit because of the increased stability in filling or half filling a large d subshell. Note that electron configurations do not have to be from the lowest to the highest energy subshells, but they usually are. |
|
|
|
What is the definition of electrostatic energy (electrostatic potential energy)?
|
In case the electric charge of an object can be assumed to be at rest, it has potential energy due to its position relative to other charged objects.
The electrostatic potential energy is the energy of an electrically charged particle (at rest) in an electric field. Electric field has units N/C because Electric field = F/q. Electric charge is quantized, therefore not continuously divisible. Electric charge it comes in multiples of individual small units called the elementary charge, e, approximately equal to 1.602×10−19 coulombs. The proton has a charge of e, and the electron has a charge of −e. Charge comes in multiples of an indivisible unit of charge, represented by the letter e. In other words, charge comes in multiples of the charge on the electron or the proton. Putting "charge is quantized" in terms of an equation, we say: q = n e q is the symbol used to represent charge, while n is a positive or negative integer, and e is the electronic charge, 1.60 x 10-19 Coulombs. 'n' is for the moles of charges there are (doubly charged, singly charged, etc). |
|
|
|
What is the definition of electrostatic force?
|
Electrostatic force, or Coulomb's law, describes the electrostatic interaction between electrically charged particles. One of the mysteries of the atom is that the electron and the nucleus attract each other. This attraction is called electrostatic force, the force that holds the electron in orbit. Like charges repel, opposite charges attract. The positive charge exerts an invisible, attractive force on the electron -- an electric force.the electric force is like an invisible spring, but as the charges move farther apart, a weaker spring pulls them together.
|
|
|
|
Determine the formal charge of ammonium.
|
Ammonium NH4+ is a cationic species. By using the vertical groups of the atoms on the periodic table it is possible to determine that each hydrogen contributes 1 electron, the nitrogen contributes 5 electrons, and the charge of +1 means that 1 electron is absent. The final total is 8 total electrons (1 × 4 + 5 − 1). Drawing the Lewis structure gives an sp3 (4 bonds) hybridized nitrogen atom surrounded by hydrogen. There are no lone pairs of electrons left. Thus, using the definition of formal charge, hydrogen has a formal charge of zero (1- (0 + ½ × 2)) and nitrogen has a formal charge of +1 (5− (0 + ½ × 8)). After adding up all the formal charges throughout the molecule the result is a total formal charge of +1, consistent with the charge of the molecule given in the first place.
Note: The total formal charge in a molecule should be as close to zero as possible, with as few charges on the molecule as possible |
|
|
|
Determine the formal charge of carbon dioxide.
|
Example: CO2 is a neutral molecule with 16 total valence electrons. There are three different ways to draw the Lewis structure
Carbon single bonded to both oxygen atoms (carbon = +2, oxygens = -1 each, total formal charge = 0) Carbon single bonded to one oxygen and double bonded to another (carbon = +1, oxygendouble = 0, oxygensingle = −1, total formal charge = 0) Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge =0) Even though all three structures gave us a total charge of zero, the final structure is the superior one because there are no charges in the molecule at all. |
|
|
|
What is the definition of Boyle's Law?
|
Boyle's Law is one of several gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system.
|
|
|
|
What is the definition of Charles's Law?
|
Charles' law (also known as the law of volumes) is an experimental gas law which describes how gasses tend to expand when heated. At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. the gas expands as the temperature increases).
|
|
|
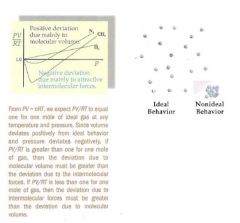
What is the definition of Van der Waals equation?
|
The van der Waals equation is an equation of state for a fluid composed of particles that have a non-zero size and a pairwise attractive inter-particle force (such as the van der Waals force.) An equation of state is a relation between state variables. It is a modification of the ideal gas law and it approximates the behavior of real fluids, taking into account the nonzero size of molecules and the attraction between them. A state variable is one of the set of variables that describe the "state" of a dynamical system. Intuitively, the state of a system describes enough about the system to determine its future behaviour.
|
|
|
|
What is the definition of Dalton's Law?
|
Dalton's law (also called Dalton's law of partial pressures) states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture. It is related to the ideal gas law.Dalton's law is not exactly followed by real gases.
|
|
|

What is the definition of Vapor pressure lowering (Raoult's law)?
|
The vapor pressure of an ideal solution (solute and solvent have similar properties such as with bonding and size) is dependent on the vapor pressure of each chemical component and the mole fraction of the component present in the solution. In chemistry, an ideal solution or ideal mixture is a solution in which the enthalpy of solution (or "enthalpy of mixing") is zero, the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the solution becomes. Ideality of solutions is analogous to ideality for gases, with the important difference that intermolecular interactions in liquids are strong and can not simply be neglected as they can for ideal gases. Instead we assume that the mean strength of the interactions are the same between all the molecules of the solution. More formally, for a mix of molecules of A and B, the interactions between unlike neighbors (UAB) and like neighbors UAA and UBB must be of the same average strength i.e. 2UAB=UAA+ UBB and the longer-range interactions must be nil (or at least indistinguishable). If the molecular forces are the same between AA, AB and BB, i.e. UAB=UAA=UBB, then the solution is automatically ideal. If the molecules are almost identical chemically, e.g. 1-butanol and 2-butanol, then the solution will be ideal. Since the interaction energies between A and B are the same, it follows that there is no overall energy (enthalpy) change when the substances are mixed. The more dissimilar the nature of A and B, the more strongly the solution is expected to deviate from ideality. Therefore, solvent-solute interactions are similar to solute-solute and solvent-solvent interactions.
|
When a nonvolatile solute is added to a liquid, some of those solute molecules will reach the surface of the solution, and reduce the amount of surface area available for the liquid molecules. Since the solute molecules don't break free of the solution but do take up surface area, the number of molecules breaking free from the liquid is decreased, while the surface area of the solution and the volume of the open space above the solution remain the same. From the ideal gas law PV = nRT, a decrease in moles (n) at constant volume and temperature is proportional to a decrease in pressure (P). The vapor pressure of a solution is given by Raoult's law and is proportional to the mole fraction of the liquid and the vapor pressure of the pure liquid.
|
|
|
What are the limitations of Raoult's law?
|
This law is strictly valid only under the assumption that the chemical interactions between the two liquids is equal to the bonding within the liquids: the conditions of an ideal solution (solute and solvent have similar properties such as with bonding and size). Therefore, comparing actual measured vapor pressures to predicted values from Raoult's law allows information about the relative strength of bonding between liquids to be obtained. If the measured value of vapor pressure is less than the predicted value, fewer molecules have left the solution than expected. This is put down to the strength of bonding between the liquids being greater than the bonding within the individual liquids, so fewer molecules have enough energy to leave the solution. Conversely, if the vapor pressure is greater than the predicted value more molecules have left the solution than expected, due to the bonding between the liquids being less strong than the bonding within each. Raoult’s law is similar in that it assumes that the physical properties of the components are identical. The more similar the components are, the more their behavior approaches that described by Raoult’s law. In an ideal solution, the bonds formed are similar to the bonds broken resulting in a zero heat of solution. High altitudes have lower atmospheric pressure. Liquid boils when its vapor pressure equals the environmental pressure surrounding the liquid. So liquid boils at a lower temp if the atmospheric pressure is lower. If you were to boil an egg in a high altitude, it would boil at a lower temperature, but would take longer to cook because it needs to cook at a higher temperature. Cooking food temperature and boiling temperature are two different things.
|
If the solution is not ideal, the intermolecular forces between the molecules will be changed. Either less energy or more energy will be required for molecules to break the intermolecular bonds and leave the surface of the solution. So the vapor pressure of a NONIDEAL solution will deviate rom the prediction made by Raoult's law. If the heat of solution (enthalpy) is negative, stronger bonds are formed, fewer molecules are able to overcome the higher energy of the stronger bonds and break free from the surface and there will be a negative deviation of the vapor pressure from Raoult's law. The predicted vapor pressure will be higher than the actual vapor pressure. Vapor pressure is lowered because of these stronger bonds. For a positive heat of solution, weaker bonds are formed, so there will be a positive deviation of the vapor pressure from Raoult's law. The actual vapor pressure will be higher than the predicted vapor pressure. Weaker bonds raise the vapor pressure because more molecules are able to overcome the energy of the weak bonds to break free from the surface. The vapor pressure of nonideal solutions is indicative of the strength of the intermolecular forces between the molecules.
|
|
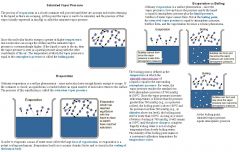
What is the definition of boiling-point elevation?
|

Boiling-point elevation describes the phenomenon that the boiling point of an ideally dilute solution will be higher when another compound is added, meaning that a solution has a higher boiling point than the pure solvent. This happens whenever a non-volatile solute (a solute which has little tendency to escape from the solution, such as a salt), is added to a pure solvent, such as water. This result also happens with vapor pressure lowering, because boiling point elevation and vapor pressure lowering mean the same thing. The result is that in dilute ideal solutions (where the solute molecules are completely separated by solvent molecules so that they have no interaction with each other), the extent of boiling-point elevation is directly proportional to the molal concentration of the solution according to the equation. Increasing the pressure on top of a liquid (like increasing the vapor pressure) increases the boiling point, while decreasing the pressure on top of the liquid (decreasing the vapor pressure) lowers the boiling point. So if you are measuring the boiling point of a liquid and a leak happens in the measurement, the pressure increased on the liquid, so the boiling point is increased, because now the liquid is harder to boil. ΔT = (kb)(m)(i), where kb is the specific constant of the substance being boiled, m is the molality of the solution, and i is the van't Hoff factor, the number of particles into which a single solute particle will dissociate when added to solution.
|
Thee boiling point of a solution is a colligative property, one which depends only on the NUMBER of solute particles, not the type. If a solute dissociates into more ions than another solute, than that solute will have a higher boiling point. Boiling point doesn't have to do with whether or not a solute is ionic.
|
|
|
What is the definition of freezing-point depression?
|

Freezing-point depression describes the phenomenon in which the freezing point of a liquid (a solvent) is depressed when another compound is added, meaning that a solution has a lower freezing point than a pure solvent. This happens whenever a solute is added to a pure solvent, such as water. The phenomenon may be observed in sea water, which due to its salt content remains liquid at temperatures below 0 °C, the freezing point of pure water. The phenomenon of boiling point elevation is analogous to freezing point depression. The result is that in dilute ideal solutions (where the solute molecules are completely separated by solvent molecules so that they have no interaction with each other), the extent of freezing-point depression is directly proportional to the molal concentration of the solution according to the equation.
|
|
|
|
Explain how freezing point depression and boiling point elevation are colligative properties.
|
They both are colligative properties, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. It is an effect of the dilution of the solvent in the presence of a solute. They are a phenomenon that happens for all solutes in all solutions, even in ideal solutions (solute and solvent have similar properties such as with bonding and size), and does not depend on any specific solute-solvent interactions. The freezing point depression and boiling point elevation happen both when the solute is an electrolyte, such as various salts, and a nonelectrolyte.
Molality is usually used in formulae for colligative properties. The boiling point elevation equation applies to an ideally dilute solution due to the addition of a NONVOLATILE solute. You cannot apply the boiling point elevation equation to VOLATILE solutes. A volatile solute can actually DECREASE the boiling point, INCREASING the vapor pressure. If you know the heat of solution (ΔH), you can make qualitative predictions about the boiling point change. For instance, since you know that an endothermic heat of solution (+ΔH) indicates weaker bonds, which means that it will be easier to boil - so the boiling point goes down, which means that the vapor pressure increases. |
When using the nonvolatile solute equations, be sure to consider the number of particles after dissociation. For boiling point and freezing point calculations, molality is used instead of molarity because molality doesn't change with temperature while molarity does. Melting point also changes when a solute is added, but it is not related to the vapor pressure. Instead, it is a factor of crystallization. Impurities (the solute) interrupt the crystal lattice and lower the freezing point.
|
|
|
What is the definition of osmotic pressure?
|

The van 't Hoff factor i accounts for the number of individual particles (typically ions) formed by a compound in solution. For most non-electrolytes dissolved in water, the van' t Hoff factor is essentially 1. For most ionic compounds dissolved in water, the factor is equal to how many ions it dissociates into in solution (eg. i = 2 for sodium chloride in water, due to dissociation of NaCl into Na+ and Cl-). This equation gives the pressure on one side of the membrane; the total pressure on the membrane is given by the difference between the pressures on the two sides. Note the similarity of the above formula to the ideal gas law.
Osmotic pressure is a measure of the tendency of water (or some other solvent) to move into a solution via osmosis. The extra pressure on the solution side is called osmotic pressure. Osmotic pressure is only relevant when comparing one solution with another - it is not really pressure at all, not like pascals or atmospheric pressure. |
High osmotic pressure corresponds to a high molarity. A high molarity means many particles per gram of substance (mol/g) placed into the solution. Thus, a high osmotic pressure means a low molecular weight (g/mol). To demonstrate osmotic pressure, we have a pure liquid move to the solution side (the side with the solute that can't cross the semi-permeable membrane). As it does, the solution level rises and the PRESSURE INCREASES. The extra pressure from the WATER moving to the SOLUTE SIDE (solution side) is the osmotic pressure.
|
|
|
How does osmotic pressure affect cells?
|
Osmotic pressure is an important factor affecting cells. Osmoregulation is the homeostasis mechanism of an organism to reach balance in osmotic pressure.
Hypertonicity is the presence of a solution that causes cells to shrink. Hypotonicity is the presence of a solution that causes cells to swell. Isotonic is the presence of a solution that produces no change in cell volume. When a biological cell is in a hypotonic environment, the cell interior accumulates water, water flows across the cell membrane into the cell, causing it to expand. |
|
|
|
How can you classify colloids?
|
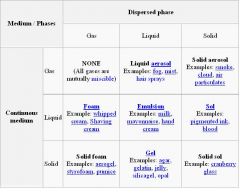
A colloid is a type of mixture in which one substance is dispersed evenly throughout another. A colloidal system consists of two separate phases: a dispersed phase (or internal phase) and a continuous phase (or dispersion medium). A colloidal system may be solid, liquid, or gaseous. Because the size of the dispersed phase may be difficult to measure, and because colloids have the appearance of solutions, colloids are sometimes identified and characterized by their physico-chemical and transport properties. For example, if a colloid consists of a solid phase dispersed in a liquid, the solid particles will not diffuse through a membrane, whereas with a solution the dissolved ions or molecules will diffuse through a membrane. In some cases, a colloid can be considered as a homogeneous mixture. A sol is a colloidal suspension of solid particles in a liquid. A colloidal system can be any combination of phases except gas in gas.
|
Unlike a true solution, colloidal suspensions will scatter light, a phenomenon known as the Tyndall effect. Colloidal particles may be attracted (lyophilic) or repelled (lyophobic) by their dispersion medium. The dispersion medium in a colloid is analogous to the solvent in a solution. Lyophobic colloids form when amphipatic or charged particles adsorb to the surface of the colloidal particles stabilizing them in the dispersion medium.
|
|

What is Henry's Law?
|

Henry's law is one of the gas laws and states that at a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. Henry's law demonstrates that the solubility of a gas is proportional to its partial vapor pressure.
An equivalent way of stating the law is that the SOLUBILITY of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid. Henry's law has since been shown to apply for a wide range of dilute solutions, not merely those of gases. An everyday example of Henry's law is given by carbonated soft drinks. Before the bottle or can is opened, the gas above the drink is almost pure carbon dioxide at a pressure slightly higher than atmospheric pressure. The drink itself contains dissolved carbon dioxide. When the bottle or can is opened, some of this gas escapes, giving the characteristic hiss (or "pop" in the case of a champagne bottle). Because the pressure above the liquid is now lower, some of the dissolved carbon dioxide comes out of solution as bubbles. If a glass of the drink is left in the open, the concentration of carbon dioxide in solution will come into equilibrium with the carbon dioxide in the air, and the drink will go "flat". The solubility of gases typically decrease with temperature (as opposed to salts which increase in temperature), because gases gain more energy and break the intermolecular bonds of the solution to be free and increase in concentration as a gas in atmosphere instead of gas in solution. |
As shown by Raoult's law and Henry's law, the partial vapor pressure of a solution component is always proportional to its mole fraction. If the component predominates as the solvent, then it is as if the solvent is an ideal solution, and Raoult's law says that the partial vapor pressure is proportional to the pure vapor pressure. If the component represents a tiny amount of solution, then this represents an ideally dilute solution, and Henry's law says that the vapor partial pressure is proportional to Henry's law constant. In an ideally dilute solution, the solvent obey's Raoult's law and the solute obey's Henry's law. Henry's law is C = k(Pv), where C is the solubility of a gas (usually in moles per liter), k is Henry's law constant, and Pv is the PARTIAL vapor pressure of the GAS above the solution, NOT the VAPOR PRESSURE of the PURE solvent, as it would be with Raoult's law. Also, Henry's law can be written as Pv = x(k), where Pv is the partial vapor pressure of the gas, x is the mole fraction of the gas in solution, and k is Henry's law constant.
|
|
|
Explain the rule of the mole concept: The chemical formula represents a mole of that substance.
|
Remember that any number placed to the left of a chemical symbol or formula is called the COEFFICIENT. This number (integer, decimal, or in scientific notation) tells us the number of moles of that substance.
Examples: Pb --> 1 mole of lead atoms (understood 1) 3 Pb --> 3 moles of lead atoms |
|
|
|
Explain the rule of the mole concept: The formula mass, expressed in grams, represents the mass of one mole of that substance.
|
The formula mass of an element is its atomic mass (found on Periodic Table.) The formula mass of a compound is found by multiplying the number of `moles' of that element (see its subscript in the formula) by that atom's atomic mass. Then add masses of all elements and record in grams.
The following example is given to demonstrate how to find formula mass. CaCO3 (calcium carbonate) Ca 1 x 40.1 = 40.1 C 1 x 12.0 = 12.0 O 3 x 16.0 = 48.0 ---- 100.1 grams Now for some examples involving this rule: 2 Cu --> 2 moles of copper atoms --> 2 x 63.5 = 127 5.00 NaCl --> 5 moles sodium chloride --> 5.00 x 58.4 = 292 g |
|
|
|
Explain the rule of the mole concept: One mole of any substance contains 6.02 E 23 particles.
|
Particles here might mean atoms, molecules, ions, electrons, or just about anything you might need to work with. Remember: just as there are 12 items in a dozen; 6.02 E 23 particles in a mole.
Examples: HNO3 --> 1 mole of nitric acid, 1.00 x 63.0 = 63.0 grams, 6.02 E 23 molecules of nitric acid 3.00 K --> 3.00 moles of potassium atoms , 3.00 x 39.1 = 117 grams, 3.00 x 6.02 E 23 =1.81 E 24 potassium atoms |
|
|
|
Explain the rule of the mole concept: One mole of any gas, at STP conditions, occupies 22.4 L of volume.
|

STP is a shorthand way of requiring the temperature to be at 0° C and a standard pressure of 1 atm (101.3 kPa).
This rule is most commonly used when studying gas laws. Suppose you have 4 grams of helium gas. This represents 1 mole of helium. These 6.02 E 23 atoms of helium would take up 22.4 liters of volume. This large volume would be fully occupied if the temperature was 0° Celsius and the pressure 1 atmosphere. A change in the temperature/pressure would, of course, change the volume occupied by the gas. |
|
|
|
What is the theoretical yield for 3Xred
3Xox + Ared → 3Xred + Aox if you react 60 grams of Xox with 63 grams of Ared given that the molecular weight of Xox is 2 amu, Ared is 7 amu, and Xred is 10 amu? |
First, find who's the limiting reagent.
Using the method described above in the limiting reactant section, we find out that Ared is the limiting reactant. Next, take the amount in mols of the limiting reactant (9 mols according the the above calculation) and do the stoichiometry to get to how many mols of 3Xred this will yield. 9 mols of Ared * 3 mols of Xred per 1 mol of Ared = 27 mols. Lastly, convert mols to grams: 27 mols * 10 g/mol = 270 g The theoretical yield for the above reaction is 270 g of Xred Say you did an actual experiment of the above reaction and you managed to obtain 243 g Xred, then the experimental yield is 243 g. Percent yield = experimental yield / theoretical yield x 100 For the above experiment, the percent yield would be 243 / 270 x 100 = 90 % |
|
|
|
What is a P-V Diagram?
|
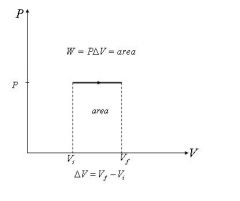
A P-V diagram describes that work done by the system= negative area under or enclosed by curve. It describes a thermal cycle following two variables, volume (x-axis) and pressure (y-axis). W = PΔV. If volume increases, then work is done on the surroundings by the system, so negative work is done on the system.
Some equations to memorize: The sum total of the kinetic and potential energy at the molecular level is called the internal energy of the system: E = KE+PE (molecular level). In an ideal gas, the interaction energy between molecules is neglected, that is, there is no internal potential energy. This means that the internal energy of an ideal gas is the sum total of the kinetic energy of the molecules. A standard derivation in most textbooks based on kinetic molecular theory relates the average kinetic energy of a molecule to the temperature: Average KE = (3/2)kT. k is Boltzmann's constant and T is in Kelvins. For an ideal gas containing n moles: E = (3/2)nRT. The formula E = (3/2)nRT is only true for a monoatomic ideal gas. To solve equations, you can use PV=nRT. E = (3/2)PV. To find the net work of a system on from a P-V diagram, it is the area under the curve. So, if the graph is broken up into squares, the best way to calculate this is to multiply volume (x) by pressure (y) that encompasses the area of interest and divide by the number of squares that that area covers. That number is the work per square. Then count the number of squares under the curve that we are interested in and multiply it by the work per square, and you have the area under the curve (the net work). Remember that the area under the curve is the TOTAL area under the curve - so make sure to calculate all the way to the x axis. |
|
|

What is an isothermal process?
|
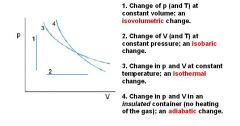
An isothermal process is one that occurs at constant temperature. Since the temperature doesn't change during an isothermal process for gases, there is no change in internal energy. The first law then tells you that the work done by the gas is just equal to the heat that flows into the system from the bath. ◦PV graph is a rectangular hyperbola
ΔE = 0 and Q = −W. However, liquids may also be isothermal, meaning no temperature change, but their internal energy depends on more than just temperature, so their internal energy, work, and heat is not zero. For a gas, internal energy only depends on temperature, that is why for isothermal processes, internal energy is zero for gases. For an isothermal system, the heat flow equals the work, the heat flow in equals the work that goes out (work done by the system). |
|
|
|
What is an isobaric process?
|
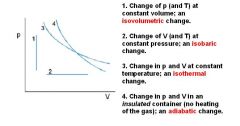
While the gas expands, the pressure P remains constant, an isobaric process. The work done by the gas during the expansion is given by: W = −PΔV and ΔU = Q − PΔV.
◦PV graph is a horizontal line |
|
|
|
What is an isovolumetric process?
|
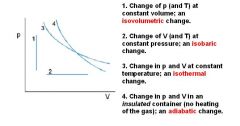
A process of constant volume and changing pressure. PV graph is a vertical line. W = 0 and ΔU = Q.
|
|
|
|
What is an adiabatic process?
|
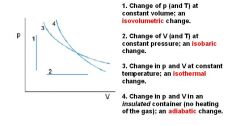
An adiabatic process is one that occurs without the exchange of heat with the surroundings. Since Q = 0 for an adiabatic process, the first law tells you that the change in internal energy is just equal to the work done on the system. When a gas expands adiabatically, the work done on the system in the expansion comes at the expense of the internal energy of the gas, causing the temperature of the gas to drop. ◦PV diagram is a "steep hyperbola". Q = 0 and ΔU = W. If there is no energy change, then ΔU = 0, so work = 0 then too. The point is, with an adiabatic system, q = 0 - there is no exchange of heat. If an ideal gas undergoes an adiabatic expansion and it is insulated, not only is there no exchange in heat, but there is also no exchange of temperature. So, because there is no ΔT, ΔU = 0 and because there is no heat exchange, q = 0, so w = 0. Therefore, the only thing that changes is pressure from the expansion (not internal energy, not temperature). For a real gas undergoing an adiabatic expansion and that is also insulated, ΔU = 0 as well, but for real gases, their behavior deviates and the gas molecules attract one another. They undergo inelastic collisions which reduce the kinetic energy. Because KE is reduced, temperature is reduced. So real gases do have a ΔT and they also have a Δ in pressure from the expansion. In adiabatic compression, temperature increases, in adiabatic expansion, temperature decreases.
|
|
|

What is rate law?
|

The rate law or rate equation for a chemical reaction is an equation which links the reaction rate with concentrations of reactants. Rate law can be determined by measuring the initial rate of the reaction for a variety of reactant concentrations, graphing the concentration of the reactants as a function of time, and finding the mechanism of the reaction. A reaction mechanism is a collection of elementary processes or steps (also called elementary steps) that explains how the overall reaction proceeds. A mechanism is a proposal from which you can work out a rate law that agrees with the observed rate laws. The fact that a mechanism explains the experimental results is not a proof that the mechanism is correct. If several steps are involved in an overall chemical reaction, the slowest step limits the rate of the reaction. Thus, a slow step is called a rate determining step. This step is used to find the rate law. Rate laws cannot be determined from the balanced equation unless the reaction is known to take place in a single step or rate determining step. To find the orders of the species, you look at the rate law box and find a reactant species who's concentration doesn't change. That's the control. The other species involved is used to compare the concentration changes to the rate changes. So say a species' concentration triples and the rate increases by 9, then the equation to find the order of that species is 3^2 = 9, so the order is 2. Likewise, if the concentration of a species doubles and the rate doubles, then you use the equation 2^1=2, so the order is 1. If you see a rate law with a fraction as an order, then that means that that reaction does not occur in a single step. More than one mechanism can generate the same rate law, so even if you have a determined a rate law experimentally, you could have multiple mechanisms of the reaction, while if you know you have the correct mechanism, then you can use that mechanism to derive the rate law.
|
Notice that the rate may be increased by increasing the concentration of the reactants. If we consider the collision model, this makes sense. The greater the concentration of a species, the more likely are collisions. When solving rate law equations, make sure to solve for k, the rate constant.
|
|

What is the Arrhenius equation?
|
◦When activation energy approaches zero, the reaction proceeds as fast as the molecules can move and collide.
◦When temperature approaches absolute zero, reaction rate approaches zero because molecular motion approaches zero. The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the rate constant, and therefore, rate of a chemical reaction. |
|
|

What is the Law of Mass Action?
|

◦The Law of Mass Action is the basis for the equilibrium constant for reversible chemical reactions.
◦What the Law of Mass Action says is basically, the rate of a reaction depends only on the concentration of the pertinent substances participating in the reaction. In chemistry, the law of mass action is a mathematical model that explains and predicts behaviors of solutions in dynamic equilibrium. It can be described with two aspects: 1) the equilibrium aspect, concerning the composition of a reaction mixture at equilibrium and 2) the kinetic aspect concerning the rate equations for elementary reactions. |
|
|
|
What are the two ways of deriving the equilibrium constant (Keq)?
|

|
|
|

What is the equilibrium constant (Keq)?
|

The Equilibrium Constant relates to a chemical reaction at equilibrium. It can be calculated if the equilibrium concentration of each reactant and product in a reaction at equilibrium is known. When pure substances (liquids or solids) are involved in equilibria they do not appear in the equilibrium equation. Pure liquids and solids have a fixed concentration (their densities) which will always be constant, so there is no need to include that in the equilibrium constant expressions. You only want to keep things with variable concentrations in the equilibrium expression. Only gases and aqueous solutions are in the expression.
|
Like rate constants, equilibrium constants depend on temperature.
|
|
|
What is the difference between Ka and pKa?
|

|
|
|
|
What is a clue for knowing what a weak acid is?
|

|
|
|
|
What is a clue for finding a weak base?
|

|
|
|
|
What is the difference between a neutralization reaction and a hydrolysis reaction?
|

|
|
|
|
What kind of solution forms from the hydrolysis of salts from a strong acid and a strong base?
|

|
|
|
|
What kind of solution forms from the hydrolysis of salts of a strong acid and weak base?
|

|
|
|
|
What kind of solution forms from the hydrolysis of salts of a weak acid and strong base?
|

|
|
|
|
What direction does the equilibrium go to for a higher Ka or Kb value?
|
A higher Ka value indicates higher hydronium ion concentrations or greater ionization. The equilibrium position lies further to the right when Ka is higher. A higher Kb value means a higher hydroxide ion concentration or greater ionization and the equilibrium position is further to the right.
|
|
|
|
What will the solution be of the hydrolysis of salts of weak acids and weak bases?
|

|
|
|
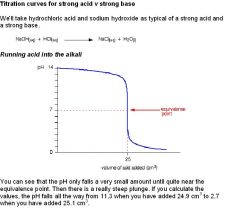
What does a titration curve look like for a strong acid and a strong base?
|
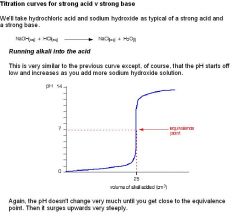
|
|
|

What is the titration curve for a strong acid and a weak base?
|
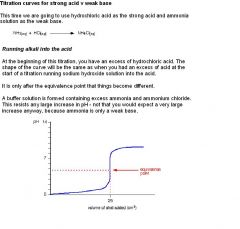
The point of inflection is the spot where we could add the largest amount of acid or base with the least amount of change in pH. Such a solution is considered buffered. The point of inflection shows the point in the titration where the solution is the most well buffered.
|
The point of inflection (half equivalence point) is where the concentration of the acid is equal to the concentration of its conjugate base, and pH = pKa.
|
|
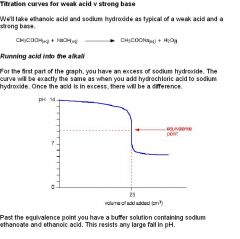
What is the titration for weak acids and strong bases?
|
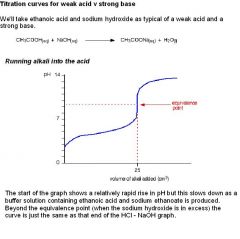
In figuring out the pH or pOH of the initial acid or base, check the graph. Look for the pH or pOH at the x = 0 axis, before the next acid or base was added. Use that value to find the concentration of H+ or OH-.
|
|
|
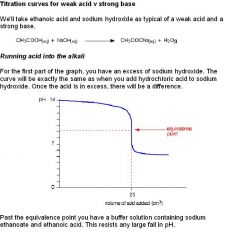
What is the titration for weak acids and strong bases?
|
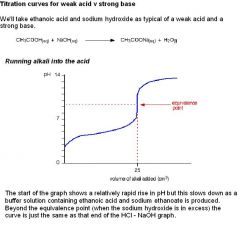
|
|
|
|
What is the titration curve for weak acids and weak bases?
|

|
|
|
|
What is the point of inflection for titration curves?
|
In monoprotic acids, the inflection point is the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence point is significant: at that point, the concentrations of the two species (the acid and conjugate base) are equal. For polyprotic acids, the first acid dissociation constant occurs at the inflection point halfway between the beginning of the curve (no titrant added) and the first equivalence point. The second acid dissociation constant (where the 2nd point of inflection occurs), however, is the point halfway between the first equivalence point and the second equivalence point. The point of inflection is where the graph changes concavity.
|
|
|
|
When does maximum buffering capacity occur?
|
Buffer systems formed by weak acids have maximum buffering capacity at the pH = pKa of the acid. This is the point of inflection.
■When [acid] = [conjugate base], the system is buffered at pH = pKa of the acid (the point of inflection). ■Buffer systems formed by weak bases have maximum buffering capacity at the pH = pKa. The pKa corresponds to the conjugate acid. ■When [base] = [conjugate acid], the system is buffered at pH = pKa. This is the point of inflection. pKa is relative to the conjugate acid. |
|
|

What is the equivalence point for titration curves?
|

The equivalence point of a chemical reaction occurs during a chemical titration when the amount of titrant added is equivalent, or equal, to the amount of analyte present in the sample. It has nothing to do with calculating pKa. In some cases there are multiple equivalence points which are multiples of the first equivalent point, such as in the titration of a diprotic acid.
|
At intermediate pH's the zwitterion concentration increases, and at a characteristic pH, called the isoelectric point (pI), the negatively and positively charged molecular species are present in equal concentration. This behavior is general for simple (difunctional) amino acids. The pI is the pH at the first equivalence point because that is when the concentration of the amino acid is equal to the concentration of the titrant. That is when the concentration of acids equals the concentration of bases. For amino acids, they are in the zwitterion form at the equivalence point.
|
|
|
What are the main components to achieve electrolysis?
|
Electrolysis is the passage of an electric current through an ionic substance that is either molten or dissolved in a suitable solvent, resulting in chemical reactions at the electrodes and separation of materials. The main components required to achieve electrolysis are: A liquid containing mobile ions - an electrolyte, an external source of direct electric current, and two solid rods or plates known as electrodes. Molten salts such as sodium chloride are also electrolytes. When driven by an external voltage applied to the electrodes, the electrolyte provides ions that flow to and from the electrodes, where redox reactions can take place. Only for an external electrical potential (i.e. voltage) of the correct polarity and large enough magnitude can an electrolytic cell decompose a normally stable, or inert chemical compound in the solution.
|
|
|
|
What is the process of electrolysis?
|
The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons from the external circuit. A liquid containing mobile ions (electrolyte) is produced by solvation or reaction of an ionic compound with a solvent (such as an acid) to produce mobile ions and an ionic compound is melted (fused) by heating. An electrical potential is applied across a pair of electrodes immersed in the electrolyte. Each electrode attracts ions that are of the opposite charge. Positively-charged ions (cations) move towards the electron-providing (negative) cathode, whereas negatively-charged ions (anions) move towards the positive anode. At the electrodes, electrons are absorbed or released by the atoms and ions. Those atoms that gain or lose electrons to become charged ions pass into the electrolyte. Those ions that gain or lose electrons to become uncharged atoms separate from the electrolyte. The formation of uncharged atoms from ions is called discharging. The energy required to cause the ions to migrate to the electrodes, and the energy to cause the change in ionic state, is provided by the external source of electrical potential.
|
|
|
|
What are galvanic cells?
|
Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. A Galvanic cell is an electrochemical cell that derives electrical energy from chemical reactions taking place within the cell. It generally consists of two different metals connected by a salt bridge, or individual half-cells separated by a porous membrane. It is sometimes called a "Voltaic cell". The key to gathering the electron flow is to separate the oxidation and reduction half-reactions, connecting them by a wire, so that the electrons must flow through that wire. That electron flow, called a current, can be sent through a circuit which could be part of any number of electrical devices such as radios, televisions, watches, etc.
|
|
|
|
What are electrolytic cells?
|
An electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis.
The concept of reversing the direction of the spontaneous reaction in a galvanic cell through the input of electricity is at the heart of the idea of electrolysis. Electrolytic cells, like galvanic cells, are composed of two half-cells--one is a reduction half-cell, the other is an oxidation half-cell. Though the direction of electron flow in electrolytic cells may be reversed from the direction of spontaneous electron flow in galvanic cells, the definition of both cathode and anode remain the same--reduction takes place at the cathode and oxidation occurs at the anode.Because the directions of both half-reactions have been reversed, the sign, but not the magnitude, of the cell potential has been reversed. |
|
|
|
How is it possible to make a non-spontaneous reaction proceed in an electrolytic cell?
|
The answer is that the electrolytic cell reaction is not the only one occurring in the system-the battery is a spontaneous redox reaction. By Hess's Law, we can sum the ΔG of the battery and the electrolytic cell to arrive at the ΔG for the overall process. As long as that ΔG for the overall reaction is negative, the system of the battery and the electrolytic cell will continue to function. The condition for ΔG being negative for the system (you should prove this for yourself) is that Ebattery is greater than - Ecell.
|
|
|
|
What does Faraday's 1st Law of Electrolysis state and how do you calculate the quantity of electricity or charge contained in a current running for a specified time?
|
The quantity of a substance produced by electrolysis is proprotional to the quantity of electricty used.
Current = coulomb's of charge per second. I = q/t. Q = I x t Q = quantity of electricity or charge in coulombs (C) I = current in amps (A) t = time (seconds) |
|
|
|
What is Faraday's constant?
|
The Faraday constant, F, is the quantity of electricity carried by one mole of electrons.
F = Avogadro's Number x charge on electron in coulombs F = 6.022 x 10^23/mol x 1.602192 x 10^-19 C F = 96,484 C/mol This is usually rounded off to 96,500 C mol-1 for calculations in chemistry. Electrons have an electric charge of −1.602×10^−19 coulomb, which is used as a standard unit of charge for subatomic particles. Within the limits of experimental accuracy, the electron charge is identical to the charge of a proton, but with the opposite sign. ◦Faraday's constant = coulombs of charge per mol of electron = total charge over total mols of electrons. F = q/n. |
|
|
|
How do you calculate the quantity of electricity required to deposit an amount of metal for Faraday's First law?
|
Q = n(e) x F
Q = quantity of electricity in coulombs (C) n(e) = moles of electrons F = Faraday constant = 96, 500 C/mol ◦q = It and q = nF, thus we get: ◦It = nF or ◦Current x time = mols of e- x Faraday's constant. Remember to calculate Faraday's constant, you need the mole of electrons. So if you have particles that only have 1 charge, then it's 1 mole of electrons. If you have particles that are doubly charged (2+), then you have 2 moles of electrons (because each electron is -). |
|
|
|
Explain the function of the half cells for galvanic cells?
|
A Galvanic cell consists of two half-cells. In its simplest form, each half-cell consists of a metal and a solution of a salt of the metal. The salt solution contains a cation of the metal and an anion to balance the charge on the cation. In essence the half-cell contains the metal in two oxidation states and the chemical reaction in the half-cell is an oxidation-reduction (redox) reaction. In a galvanic cell one metal is able to reduce the cation of the other and, conversely, the other cation can oxidize the first metal. The two half-cells must be physically separated so that the solutions do not mix together. A salt bridge or porous plate is used to separate the two solutions. The number of electron transferred in both directions must be the same, so the two half-cells are combined to give the whole-cell electrochemical reaction.
|
|
|
|
Where do the anions go in a galvanic cell?
|
When a metal in one half-cell is oxidized anions must be transferred into that half-cell to balance the electrical charge of the cation produced. The anions are released from the other half-cell where a cation is reduced to the metallic state. Thus, the salt bridge or porous membrane serves both to keep the solutions apart and to allow the flow of anions in the direction opposite to the flow of electrons in the wire connecting the electrodes. Positive ions move across the salt bridge to the cathode. The salt bridge is used to balance the charges. Since negative electrons move to the cathode, positive ions must balance the charge by moving to the cathode as well.
|
|
|
|
Why do galvanic cells use resistors?
|
The voltage of the Galvanic cell is the sum of the voltages of the two half-cells. It is measured by connecting a voltmeter to the two electrodes. The voltmeter has very high resistance, so the current flow is effectively negligible. Instead of the current flowing through it, the voltmeter measures the voltage attempting to flow through it from each cell, and these voltages are added together. The resistance of an object can be defined as the ratio of voltage to current. The volt is defined as the value of the voltage across a conductor when a current of one ampere dissipates one watt of power in the conductor (V = W/A) and is also equal to one joule of energy per coulomb of charge, J/C.
|
|
|
|
What do cell potentials show?
|

Whether or not a reaction will occur spontaneously.
|
|
|
|
What are intensive and extensive properties?
|
In the physical sciences, an intensive property is a physical property of a system that does not depend on the amount of mass present or the size of the system, even if the size of the system changes: it is scale invariant. Scale invariance is a feature of objects or laws that do not change if length scales (or energy scales) are multiplied by a common factor. By contrast, an extensive property of a system is directly proportional to the mass of the system and the size of the the system and it changes when the size of the system changes. Intensive properties are intrinsic to a particular subsystem and remain constant regardless of size. For example, density is an intensive quantity (it does not depend on the quantity), while mass and volume are extensive quantities. Examples of intensive properties include: anything divided by moles or grams, like molar heat of solution or specific heat per gram, temperature, chemical potential, density, specific gravity, viscosity, velocity, electrical resistivity, specific heat capacity (specific heat), melting point and boiling point, pressure, magnetism, and concentration. Concentration is an intensive property, because no matter how much of it you have, it always has the same RATIO of solute to solution or solvent, so it doesn't depend on size of the sample. Note that the ratio of two extensive quantities is an intensive quantity. An extensive quantity is a physical quantity whose value is proportional to the size of the system it describes. Such a property can be expressed as the sum of the quantities for the separate subsystems that compose the entire system. Extensive quantities are the counterparts of intensive quantities. Dividing one type of extensive quantity by a different type of extensive quantity will in general give an intensive quantity. For example, mass (extensive) divided by volume (extensive) gives density (intensive). Examples of extensive properties include: entropy, enthalpy, energy, mass, and volume. State functions like entropy and enthalpy are properties that depend on the current of the system and they are different from intensive and extensive properties. Extensive properties are proportional to the amount of material, while intensive properties do not depend on the amount of material. Anytime you see something divided by grams, or moles, or anything - that is a ratio - and anything with ratios do not change - they are intensive properties.
|
Extensive properties are those that you can look at externally and measure the amount of it. Intensive properties are those you cannot measure.
|
|
|
What is the Heisenberg Uncertainty principle?
|

The Heisenberg Uncertainty principle arises from the dual nature (wave-particle) of matter. It states that there exists an inherent uncertainty in the product of the position of a partical and its momentum, and that this uncertainty is on the order of Planck's constant. Since we know the mass of an electron, the uncertainty must lie with the velocity and the position.
|
|
|

What is the difference between and state function and a path function?
|
There are 2 types of properties that describe the macroscopic state of a system: extensive and intensive. Intensive and extensive properties can both be state functions. If you combine 2 identical systems and a property is the same for both the single system and the combined system, that property is intensive. If a property doubles when the systems are combined, the property is extensive. If you divide one extensive property by another, the result is an intensive property (a ratio). The macroscopic state if any one-component fluid system in equilibrium can be described by just 3 properties, one of which is extensive. Path functions are not extensive or intensive properties - they do not describe the state of a system. They include work and heat.
|
|
|

How is convection related to thermal conductivity?
|
Heat can be conducted through a single object. An object's ability to conduct heat is called its thermal conductivity k. The thermal conductivity of an object depends on its composition and to a much lesser extent, its temperature. Heat divided by time is the rate of heat flow or heat current I. In a steady state, the rate of heat flow is constant any number of slabs between two heat reservoirs. In other words, if the series of slabs were lined up end to end between hot and cold reservoirs, the rate of heat flow (Q/t) would be the same in all slabs even if they had different lengths, thicknesses, and different thermal conductivities. This is similar to the flow rate in fluids and to the flow of current through resistors in series, the flow rate stays the same! Since the rate of energy transfer is the same for each slab, the ORDER in which we place the slabs does NOT affect the overall conductivity. Since the rate of heat flow is the same through each slab, a HIGHER conductivity results in a lower temperature difference across any slab of a given heat and conductivity k is inversely proportional to ΔT. Q/t = kA (ΔT/L), where ΔT is T from the hot slab minus T from the cold slab.
|
|
|

What is radiation
|
Radiation is thermal energy transfer via electromagnetic waves. All objects with a temperatuer above 0 K radiate heat. The rate at which an object RADIATES electromagnetic radiation (its power P) depends on its temperature and surface area. If we substitute the temperature of the environment for the temperature of the object, we can find the rate at which the object ABSORBS radiant heat from its environment. The environment would be hotter than the object and the object would absorb that heat. Newton's law of cooling states that the rate of cooling of a body is approximately proportional to the temperature difference between the body and its environment. An object that radiates heat faster also absorbs heat faster. When radiation strikes an opaque surface (a surface that doesn't let all light to pass through), only a fraction is absorbed. The rest is reflected. White colors reflect heat, so it conserves heat better than black colors, which absorb heat, but also radiate it back out into the environment.
|
When metal is heated, it glows red, orange-yellow, white, and finally blue-white. The hot metal radiates visible electromagnetic waves. The heat that is radiated is from electromagnetic waves. If the term RADIATE is used to describe heat, it's electromagnetic waves, even though this heat exists in the air (usually, unless it's a vacuum). The air won't be involved in transferring the energy, unless we are told that an air current (or fluid current) exists. Also, radiation is travels as quick as light.
|
|

How is work different for chemistry and physics?
|
In physics, work is an energy transfer due to a FORCE. In physics, work typically changes the position of a body. In chemistry, we are looking at work for a chemical system at rest. A system at rest may change its shape or size, but does not translate or change its position. A system at rest, with no gravitational potential energy and no kinetic energy, may still be able to do PV work. Constant pressure conditions allow us to calculate the work done using W = PΔV. Understand that PV work takes place when a gas expands against a force regardless of whether or not the pressure is constant, because P = F/A.
|
|
|
What are heat engines?
|
The second law of thermodynamics says that heat cannot be changed completely into work in a cyclical process. A machine that converts heat to work is called a heat engine. Via conservation of energy, the heat entering the engine must equal the net work done by the engine plus the heat leaving the engine. With Carnot engines, the further apart the temperatures of the two reserviors, the more effective the cyclical conversion of heat into work. If the temperatures are really close together, than there is a smaller effeciency. If the temperatures are farther apart, then the efficiency gets bigger. Carnot engines purpose is to convert heat into work, so we want the most work out of that engine. If it's not efficient enough, then less work is converted from heat, and e is closer to zero. We need a big temperature difference to get the most work out of the engine. An air conditioner and a refrigerator are like heat engine running backwards, it is creating the cold environment (cold reservoir), and needs to expel the heat that it creates into a hot reservoir. Temperature difference is directly proportional to the distance between two points of the same material for slabs between a hot and cold reservoir. The efficiency of a thermodynamic process (like a heat engine), tells you the percent of input energy (if you multiply e by 100), is converted into work. No thermodynamic process can be 100 percent efficient. So, if you are told that a steam engine has 10 percent efficiency, that means that 10 percent of the input energy (heat) contributes to the work done by the engine. The 10% is the energy input (heat) that is converted into work.
|
In other words, for efficiency, out of all of the energy that is put into an engine, if it has 10% efficiency, only 10% of all of that energy actually gets converted into work done by the engine. The reason that all energy cannot be completely converted into work is because that would cause a decrease in entropy of the universe (which can't happen).
|
|
|
What are standard states?
|
Do not confuse standard states and STP. For a pure solid or liquid, the standard state is the REFERENCE form of a substance at any chosen temperature T and at a pressure of 10^5 Pascals (1 bar). The reference form is usually the most stable at 1 bar and T. For a pure gas, the gas has to behave like an ideal gas. An ELEMENT in its standard state at 25°C is arbitrarily assigned an enthalpy value of 0 J/mol. From this value we can assign enthalpy values to COMPOUNDS based upon the change in enthalpy when they are formed from raw ELEMENTS in their standard states at 25°C. Such enthalpy values are called standard enthalpies of formation. The standard enthalpy of formation is the change in enthalpy for a reaction that creates 1 MOLE of that COMPOUND from its raw ELEMENTs in THEIR STANDARD STATES.
|
|
|
|
What are the 3 steps of the formation of a solution and what do the energy differences tell you of the stability of bonds?
|
The formation of a solution is a physical reaction. It involves 3 steps: 1) the breaking of the intermolecular bonds between solute molecules; 2) the breaking of the intermolecular bonds between solvent molecules; 3) the formation of intermolecular bonds between the solvent and the solute molecules. Energy is always required to break a bond. Since energy is required to break a bond, the 1st 2 steps in dissolution are endothermic while the 3rd step is exothermic. Since enthalpy and heat are equal at constant pressure, a solution with a negative enthalpy will give off heat (heat leaves or -q) when it forms, and a solution with positive enthalpy means heat is absorbed (+q) - all at constant pressure. Thus, a solution that gives off heat when it forms is creating stronger bonds within the solution. The new intermolecular bonds are more stabe than the old and in general, the intermolecular attractions within the solution are stronger than the intermolecular attractions within the pure substances. Remember that less energy in the system usually means a more stable system. If the overall reaction absorbs energy (is endothermic), the reverse is true.
|
The overall change in energy of the reaction is equal tot he change in enthalpy and is called the heat of solution ΔH. A negative heat of solution results in stronger intermolecular bonds, while a positive heat of solution results in weaker intermolecular bonds. Since the combined mixture is more disordered than the separated pure substances, most of the time, the FORMATION of a SOLUTION involves an increase in entropy. On the MCAT, solution formation has positive entropy.
|
|

How is vapor pressure and melting point related?
|
Boiling occurs when the vapor pressure of a liquid equals the atmospheric pressure. Melting occurs when the vapor pressure of the solid phase equals the vapor pressure of the liquid phase. The melting point is the temperature at which the vapor pressures of the solid is equal to the vapor pressure of the liquid. Above the melting point, the liquid vapor pressure is greater than that of the solid, below the melting point, the liquid vapor pressure is less than that of the solid. The vapor pressure of solution might be lower than just 1 of the pure substances but all of them in an IDEAL SOLUTION. If the vapor pressure of the solution drops below or above all of the solvents, then that solution is NONIDEAL. In an ideal solution, the vapor pressure will be somewhere in between the vapor pressures of the solute and solvent, depending on their relative mole fractions.
|
Negative heats of solution form stronger bonds and lower vapor pressure; positive heats of solution form weaker bonds and raise vapor pressure. A lower vapor pressure means more energy is needed to raise the vapor pressure to equal atmospheric pressure. Thus, a lower vapor pressure means a higher boiling point.
|
|

What is the difference between constant pressure and constant volume heat capacities?
|
If, while being heated, no PV work is done by a system at rest, nearly all the heat energy goes into increasing the temperature and all of the heat energy is kept in the system because the system doesn't expand and do work on the surroundings (ΔU = q). When the system is allowed to expand at constant pressure, some ENERGY leaves the system as work done on the surroundins and the temperature increase is diminished because all of the heat energy isn't kept in the system (ΔU = q - w). Think of it as energy being kept in the system or left. More energy kept in the system means a bigger temperature change, less energy kept in the system means a smaller temperature change.
|
|
|
|
What is the difference between evaporation and boiling and what is the difference between melting and boiling in terms of entropy and enthalpy?
|
Evaporation occurs when the PARTIAL pressure ABOVE the LIQUID is LESS than the LIQUID'S vapor pressure, but the atmopheric pressure is GREATER than the vapor pressure. Under these conditions, the liquid evaporates rather than boils. Melting and boiling are endothermic processes; heat is added to break the bonds. Melting and boiling normally increase volume and molecular movement, and therefore result in increased system entropy, so entropy and enthalpy are both positive for the processes of melting and vaporizatino, and both negative for the processes of freezing and condensing. From the equation ΔG = ΔH - TΔS, we see that when enthalpy and entropy have the same sign, temperature will dictate in which direction the reaction will move (whether or not it is spontaneous). So phase changes at constant pressure are governed by temperature.
|
|
|
|
What is the difference between the expected value and the observed value for the van't Hoff factor?
|

|
|
|
Do acids dissociate less or more in more concentrated solutions?
|
Acids dissociate less in more concentrated solutions. This does NOT mean that more concentrated solutions are less acidic. More concentrated solutions mean more acid strength.
|
|
|

How do you approximate the pH of a weak acid or base?
|

|
|
|
|
What is the Henderson-Hasselbach equation?
|

|
|
|
|
When is the concentration of the the conjugate base of the first acid of a polyprotic acid the greatest?
|

The concentration of the conjugate base of the 1st acid is greatest at the first equivalence point. At this point, it is at 100% concentration.
|
|
|
|
What is the half reaction that occurs at a standard hydrogen electrode? Do you multiply half reaction potentials when combining 2 reactions together?
|

|
|
|
|
How do you calculate the free energy of a cell potential?
|

|
|

