![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
ELECTRON STRUCTURE
|
.
|
|
|
Bohr Model
|
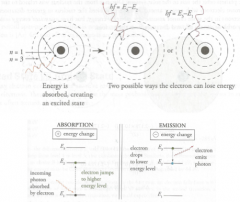
1 e- model with discrete energy (E) levels
*E in = E out (E in = E out 1 + E out 2) |
|
|
Plank's Equation
|
E = hf, f = c/lamda(wavelength)
--> E = hc/wavelength c = speed of light (3 x 10 ^8 m/s) h = plank's constant |
|
|
Electromagnetic Spectra
|
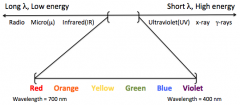
|
|
|
Quantum Model
|
Multiple e- model
Quantum numbers 1) n 2) l 3) ml 4) ms |
|
|
n
|
Shell #
n = 1, 2, 3,...n Energy lavel |
|
|
l
|
Subshell #
l = 0, 1, 2,...n-1 Shape |
|
|
ml
|
Orbital #
ml = -l to l Orientation |
|
|
ms
|
Spin #
ms = +/- 1/2 2e- per orbital |
|
|
Shapes
|

|

