![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
6 Cards in this Set
- Front
- Back
|
What is crude oil? |
The process that is used to break long-chain hydrocarbons down into shorter, more useful hydrocarbons. |
|
|
What is a fraction? |
A group of hydrocarbons that condense together when crude oil is seperated using fractional, e.g. petrol, naphtha, kerosene, etc. |
|
|
What is fractional distillation? |
A process that can be used to separate the substance in a mixture according to their boiling points |
|
|
What is crude oil made up of? |
Formed from the remains of plants and animals, mainly plankton, that died millions of years ago
Over millions of years, with high temperature and pressure, the remains turn to crude oil, which can be drilled up from the rocks where it is found. Because it takes so long for crude oil to form it's said to be a finite resource-once its used up we can't replace it
Most of the compounds in crude oil are hydrocarbon molecules, and the majority of them are alkanes |
|
|
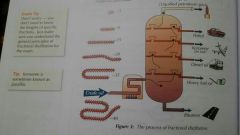
How fractional distillation is used to seperate crude oil? |
The crude oil is first heated so that it evaporates and is then piped at the bottom of the column. The gas rises up the column and gradually cools Different compounds in the mixture have different boiling points, so they condense at different temperatures. This means that they condense at different levels in the fractioning column. Hydrocarbons that have a similar number of carbon atoms have similar boiling points, so they condense at similar levels in the column. The groups of hydrocarbons that condense together are called fractions. The various fractions are constantly tapped off the column at the different levels where they condense. |
|
|
Process of fractional distillation |
|

