![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
120 Cards in this Set
- Front
- Back
|
What is a triacylglycerol?
|
TAG
fatty acids stored as TAG's |
|
|
What are 4 functions of fatty acids?
|
1. energy stores
2. building blocks for phospholipids and glycolipids sphingolipids 3. modifying proteins 4. hormones and secondary messengers |
|
|
What are the 4 steps of FA degradation?
|
oxidation
hydration oxydation cleavage |
|
|
What are the 4 steps of FA synthesis?
|
condensation
reduction dehydration reduction |
|
|
What does the degradation of a FA produce?
|
an activated acetyl unit
acetyl CoA |
|
|
What two pieces come together in FA synthesis?
|
Malonyl group and activated acyl group
|
|
|
What is an activated acyl group?
|
for our purposes it is the oxo-thioester part
O ll --C--S--R |
|
|
Describe what happens in degradation of FA.
|
activated acyl group gets oxidized (makes a double bond) between the B and gamma carbon
the double bond is hydrated to make OH and CH2 the OH gets oxidized to a ketone (C=O) coenzyme A cleaves the FA leaving it 2 carbons shorter |
|
|
What makes TAG's highly concentrated stores of metabolic energy?
|
they are REDUCED and ANHYDROUS.
|
|
|
What does oxidation of a FA give off in terms of energy?
|
9 kcal/g (vs. 4 kcal/g for carbs)
|
|
|
Where are TAG's stored?
|
cytoplasm of adipocyte
|
|
|
How are TAG's absorbed across the intestinal epithelium.
|
They are not. They have to be degraded to FA's to go across.
|
|
|
What degrades TAG's to free FA's and monoacylglycerols?
|
lipases
|
|
|
How do lipases, which in aq solution get to TAG's to degrade them?
|
bile salts --glycocholate
wrap the TAG's in a water soluable cloak and incorporate them into micelles in the lumen of the intestine. |
|
|
Why would liver disease lead to steatorrhea?
|
bile salts glycocholate are produced in the liver.
If bile acid production is decreased in liver from liver disease TAG's are not getting degraded as much and go out in the feces |
|
|
How do lipases work on the TAG's attached to micelles?
|
They hydrolyze the TAG ester bond on surface of micelle creating free FA and monoacylglycerol (MAG)
|
|
|
where are bile salts stored?
|
gall bladder
|
|
|
What happens after a TAG gets broken down into FA's and monoacylglycerol and transported over the intestinal epithelium?
|
TAG's are resynthesized and packaged into chylomicrons.
Then the chylomicron "package" is dumped into the lymph and the blood where they lazy river it around to bind to membrane lipases (usually on fat cells and muscles) that break em down again for transport into the tissue |
|
|
What are chylomicrons?
|
Big guys (200 nm) diameter
They contain TAG's, apolipoprotein B-48 they also transport fat soluable vitamins (VIT E) and cholesterol |
|
|
How do peripheral tissues gain access to adipose tissues energy stores?
|
3 stages
1). lipids mobilized (turn TAG's back into FA's and MAG's and send em out) 2). at the target tissue, FA's have to be activated and transported into mitochondria 3. In mitochondria, the FA's are broken down into acetyl CoA and then GO CITRIC ACID CYCLE!! |
|
|
what catalyzes the hydrolysis of FA's in lipids?
|
hormonall controlled lipases in adipose cells
|
|
|
what hormones control the lipases in adipose tissues?
|
epi, norepi, glucagon and ACTH
|
|
|
how would epi release lipid energy stores?
|
exercise induces epi which binds to the 7TM receptor on adipose cells. This binding activates adenylate cyclase to make cAMP
cAMP activates PKA which phosphorylates 2 key proteins perilipin A and hormone sensitive lipase |
|
|
What are the 2 key proteins in the hormone regulated lipase cascade?
|
perilipin A
hormone sensitive lipase When perilipin A gets phosphorylated it moves the TAG's into a position where hormone sensitive lipase can get to it better. And then the phosphorylated hormone sensitive lipase hydrolyzes the TAG in free FA's. |
|
|
how does the insoluable FA's freed by lipolysis in the adipose cell get around the body?
|
binds to serum albumin in the blood.
|
|
|
What are the pieces released by hormone induced lipolysis in the adipose cells?
|
glycerol and free FA's.
|
|
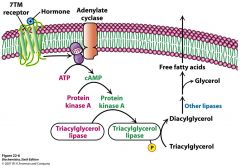
Describe this picture.
What are the hormones? Where is this taking place? |
epi, norepi, glucagon and ACTH bind to 7TM receptor and activate adenylate cyclase which makes cAMP which activates PKA which phosphorylates perilipin A and hormone sensitive lipase and degrades TAG's.
This is in the adipose cell |
|
|
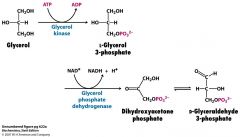
What's the fate of the glycerol produced when lipolysis occurs?
|
glycerol gets absorbed in the liver and gets phosphorylated by glycerol kinase.
Then, it gets oxidized by glycerol phosphate dehydrogenase to DHAP (then goes into glycolytic / gluconeogenic pathway) |
|
|
What are the 2 enzymes in the liver that get glycerol to the glycolytic pathway?
|
glycerol kinase (just remember glycerol is what you are dealing with)
glycerol phosphate dehydrogenase (just remember you are oxidizing the phosphorylated glycerol phosphate) If you wanna get really fancy pants remember that triose phosphate isomerase changes DHAP to GAP |
|
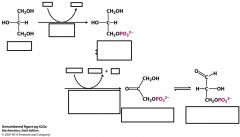
name these structures, enzymes and cofactors
|
|
|
|
What's the reaction that takes place on the outer mitochondrial membrane and what is the driving enzyme?
|
activation of a FA
formation of a thioester linkage to coenzyme A before they go inside the mito matrix enzyme = acyl CoA synthetase |
|
|
What are the 2 steps for activating FA
|
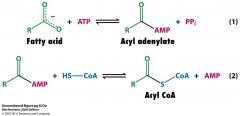
FA + ATP form mixed anhydride acyl adenylate
PPi is released and hydrolyzed by pyrophosphatase CoA-SH attacks acyl adenylate to form Acyl CoA + AMP Equivalent of 2 ATP drives overall reaction |
|
|
What does FA react with ATP to form?
|
acyl adenylate
|
|
|
because the activation reaction of FA's is highly reversible what drives the rxn forward?
|
pyrophosphatase readily hydrolyzes the pyrophosphate (2 PPi) given off after ATP makes the acyl adenylate with a FA
|
|
|
So how does an activated FA get into the mitochondrial matrix?
|
conjugates with carnitine
|
|
|
What is carnitine?
|
a zwitterionic alcohol
the acyl group transfers from Sulfur of CoA to the OH of carnitine |
|
|
Which enzyme piggy backs the acyl group of CoA onto carnitines OH?
Where does this take place? |
carnitine acyltransferase I
outer mitochondrial membrane |
|
|
what enzyme shuttles acyl carnitine from the outer mitochondrial membrane into the matrix?
|
acyl carnitine translocase
|
|
|
What enzyme catalyzes the transfer of the acyl group back to CoA in the inner mitochondrial matrix?
|
carnitine acyltransferase II
|
|
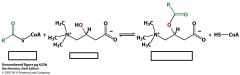
Name the structures and the enzyme that catalyzes this reaction
|
Acyl CoA
Carnitine (note the structure and where the acyl group attaches) acyl carnitine Enzyme is carnitine acyltransferase I |
|
|
What are some symptoms associated with carnitine deficiency?
|
muscle cramping and weakness
muscle , kidney and heart tissues usually most effected. |
|
|
Why is the degradation of acyl CoA called B-oxidation?
|
Because as the 4 steps occur the fatty acid is shortened by 2 carbons and the oxidation takes place at the B carbon.
|
|
|
What are the 2 oxidizing agents in B-oxidation of FA?
|
FAD
NAD+ |
|
|
What agent is responsible for the thiolysis step in B-oxidation of FA?
|
CoA
|
|
|
What enzyme catalyzes the oxidation of the B-carbon in B oxidation of FA?
|
acyl CoA dehydrogenase
|
|
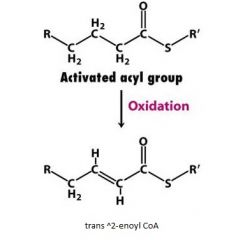
what is the enzyme that catalyzes this reaction?
|
acyl CoA dehydrogenase
Acyl CoA + E-FAD ---> trans^2enoly CoA + E-FADH2 |
|
|
What is ETF?
What does it do? |
Electron-transferring Protein
accepts electrons from FADH2 Then it gives the electrons to ETF ubiquinone reductase |
|
|
What happens when electrons are given to ETF ubiquinone reductase?
|
ubiquinone is reduced to ubiquinol and then it can do what it does in Complex III of electron transport chain
Q--->QH2 yields 1.5 ATP / FADH2 |
|
|
Please explain the flow of electrons from the first step of B-oxidation pathway
|
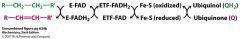
2 electrons go to
E-FADH2 to ETF-FADH2 to ETF: ubiquinone reductase where ubiquinone goes to ubiquinol Complex III in respiration!! |
|
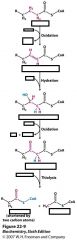
Name all this stuff!
|
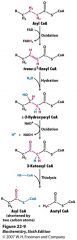
|
|
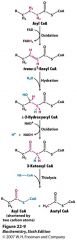
Name all the enzymes involved.
|
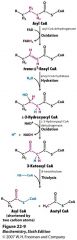
|
|
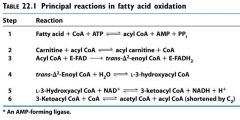
Name the enzymes
|

|
|
|
how many ATP are released from the oxidation of NADH in the respiratory chain?
|
2.5 ATP
|
|
|
How many ATP are produced from FADH2 making ubiquinol?
|
1.5 ATP
|
|
|
How many ATP are generated from acetyl CoA in citric acid cycle?
|
10 ATP
|
|
|
How many ATP are used up in the activation of FA?
|
2 ATP
This is the cost of ATP to get all the ATP out of FA degradation. |
|
|
What's the stoichiometry of the complete oxidation of palmitoyl CoA?
|
7 reaction cycles
palmitoyl CoA + 7 FAD + 7 NAD+ + 7 CoA + 7 H2O 8 acetyl CoA + 7 FADH2 + 7 NADH + 7 H+ |
|
|
How many ATP does the complete oxidation of palmitoyl CoA yield?
|
palmitoyl CoA + 7 FAD + 7 NAD+ + 7 CoA + 7 H2O 8 acetyl CoA + 7 FADH2 + 7 NADH + 7 H+
8 acetyl CoA (80 ATP), 7 FADH2 (10.5 ATP), 7 NADH (17.5 ATP) total of 108 ATP 2 ATP equivalents used to activate palmitate complete oxidation of palmitate gives 106 ATP |
|
|
What are the 2 additional enzymes needed in order to break down odd numbered or unsaturated FA's?
|
isomerase and reductase
|
|
|
What does cis-^3-enoyl CoA isomerase do?
|
This is the enzyme that palmitoleate uses when it gets degraded to a cis double bond that is not a substrate for acyl CoA dehydrogenase.
It makes the cis^3 bond a trans^2 bond and the degradation continues from there normally. |
|
|
What is a peroxisome?
|
small, membrane bound organelle which can oxidize FA's down to octanoyl CoA.
contain catalase |
|
|
How is peroxisomal oxidation different from mitochondrial B-oxidation in the 1st step?
|
No FADH2 production
flavoprotein acyl CoA dehydrogenase transfers electrons to O2 to yield H2O2 instead of transfering electrons to FADH2 as in B-oxidation Yikes, but catalase converts H2O2 to O2 and H2O. |
|
|
What must acetyl CoA combine with in order to enter into the citric acid cycle?
Where does this molecule come from? |
Oxaloacetate (OAA)
OAA comes from pyruvate, a product of glycolysis. Therefore in fasting or diabetes (when pyruvate is being used to run the gluconeogenesis pathway) there is none left over to make OAA and thus acetyl CoA has no buddy. |
|
|
What happens if there is no OAA to condense with acetyl CoA?
|
acetyl CoA is diverted to make acetoacetate and D-3-hydroxybutyrate
(ketone bodies) |
|
|
What are acetoacetate (acetone) and D-3 hydroxybutyrate?
|
ketone bodies.
produced when there is no OAA to condense with acetyl CoA in diabetes and fasting |
|
|
Where does the formation of acetone and D-3-hydroxybutyrate take place?
|
liver
|
|
|
what is the enzyme that reduces acetoacetate to D-3-hydroxybutyrate?
|
D-3-hydroxybutyrate dehydrogenase
|
|
|
What is the significance of acetone breath in an uncontrolled diabetes patient?
|
it means there are a lot of ketone bodies.
ketone bodies are produce when there is no OAA to condense with acetyl CoA so it can go into citric acid cycle. Therefore, acetoacetate and D-3-hydroxybutyrate are made and acetoacetate spontaneously decarboxylates to acetone in the blood. |
|
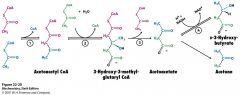
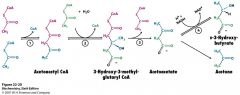
name the enzymes involved
|
|
|
|
Draw acetoacetate, D-3-hydroxybutyrate and acetone
|
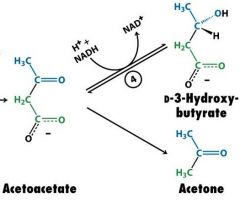
|
|
|
What tissues use acetoacetate and 3-hydroxybutyrate instead of glucose?
|
heart
renal cortex |
|
|
What tissues normally use glucose but can be trained to use ketone bodies for fuel?
|
brain
|
|
|
Since ketone bodies are a significant source of fuel how does the body get to the goodies of acetoacetone and D-3-Hydroxybutyrate?
|
acetoacetate -->CoA transferase --> acetoacetyl CoA -->thiolase--> 2 acetyl CoA
|
|
|
how do you get acetyl CoA from acetoacetate?
|
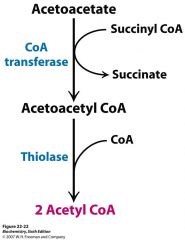
|
|
|
How do you get acetyl CoA out of 3-D-hydroxybutyrate?
|
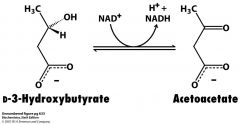
Have to do this step to get to acetoacetate and then do the break down from acetoacetate (CoA transferase) to Acetoacetyl CoA (thiolase) to 2 acetyl CoA
|
|
|
Where does FA synthesis take place?
Where does FA degradation take place? |
synthesis--in cytosol
degradation--matrix of mitochondria |
|
|
What are intermediates attached to in FA synthesis?
In FA degradation? |
synthesis--acyl carrier proteins (ACP)
degradation--acetyl CoA |
|
|
What's the difference between the enzymes in FA synthesis vs. FA oxidation?
|
In FA synthesis the enzyme is all on one Fatty acid synthase.
In FA oxidation all the enzymes are autonomous |
|
|
What's the starting block for FA synthesis?
|
malonyl CoA
|
|
|
In FA synthesis, what is the reductant used?
|
NADPH
|
|
|
Where does the elongation of FA synthesis halt?
|
C 16
palmitate |
|
|
What's the first step in FA synthesis
|
carboxylation of acetyl CoA via acetyl CoA carboxylase (isn't that name handy) to make malonyl CoA
|
|
|
What's the first step in the synthesis of a FA?
What's the enzyme responsible Draw the structures |
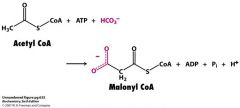
acetyl CoA carboxylase (ACC)
|
|
|
What is the activated CO2 in the committed step of FA synthesis?
|
Carboxyl group of biotin is attached to a E-lysine amino group.
1 ATP is used to get this CO2 on the biotin This is the activated CO2 that then gets transferred onto acetyl CoA to make malonyl CoA |
|
|
What is an ACP?
|
It's like a monster truck version of CoA.
Has SER-- phosphopantetheine group--SH |
|
|
What is FAS?
|
a complex of distinct enzymes that catalyze FA synthesis
Has: acetyl transacylase (AT) malonyl transacylase (MT) Acyl-malonyl ACP condensing enzyme B-ketoacyl ACP reductase 2-Hydroxyacyl ACP dehydratase enoyl ACP reductase |
|
|
What does acetyl transacylase do?
|
transfers the acetyl group from acetyl CoA to the ACP
|
|
|
What does malonyl transacylase do?
|
transfers the malonyl group from malonyl CoA to ACP forming malonyl ACP + CoA
|
|
|
Name the enzymes of FAS involved in FA synthesis
|

|
|
|
What enzyme starts FA synthesis of odd numberd FA's?
|
propionyl ACP is formed when acetyl transacylase transfers propionyl group from propionyl CoA to propionyl ACP
|
|
|
What are the 2 reactants in the condensation reaction in FA synthesis?
|
acetyl ACP (made from Acetyl CoA and AT)
malonyl ACP (made from Malonyl CoA and MT) |
|
|
What is the enzyme that condenses Malonyl ACP with Acetyl ACP?
What is the resultant product? |
The acyl-malonyl ACP condensing enzyme (see how easy the naming is here--may be a mouthful but it makes sense!!!) portion of FAS
product is acetoacetyl ACP + ACP + CO2 |
|
|
Why is the CO2 released from the condensation of malonyl ACP with Acetyl ACP significant?
|
It drives the reaction because the release of the CO2 from Malonyl ACP decreases the free energy (favorable)
|
|
|
Draw the condensation and subsequent reduction reactions in FA synthesis. Label enzymes and cofactors.
|
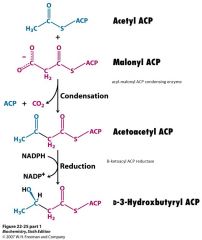
|
|
|
draw the dehydration and 2nd reduction reactions in FA synthesis. label cofactors, enzymes and structures
|
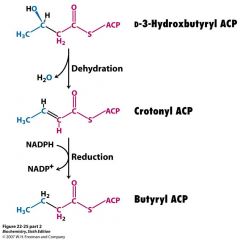
|
|
|
What's the general principle with regard to NAD+ and NADPH in biosynthetic reactions?
|
NAD+ is the oxidizing agent
NADPH is the reducing agent therefore NADPH is consumed in biosynthetic reaction and NADH is released. |
|
|
What are the starting blocks for the second round of fatty acid synthesis?
|
malonyl ACP and butyryl ACP
|
|
|
What's the overall stoichiometry of palmitate?
|
8 acetyl CoA + 7 ATP + 14 NADPH + 6 H+ ---> palmitate + 14 NADP+ + 8 CoA + 6 H2O + 7 ADP+ + 7Pi
|
|
|
Describe mammalian FAS?
|
Huge homodimer 272 kDa subunits
3 domains connectd by flexible regions. |
|
|
What happens in domain 1 of FAS?
|
substrate entrance and where the condensation action occurs (AT, MT, CE)
|
|
|
How many acetyl CoA are needed for the synthesis of palmitate?
|
Need 8 acetyl CoA
|
|
|
Where is acetyl CoA formed?
Where does FA synthesis occur? How do they get together? |
acetyl CoA is formed in mitochondrial matrix
FA synthesis takes place in cytosol acetyl CoA piggy backs on OAA to make citrate which is transported to cytosol when there is a lot Then ATP-citrate lyase in the cytosol breaks up citrate at the expense of ATP hydrolysis |
|
|
What is ATP-citrate lyase?
|
The enzyme that breaks down citrate in the cytosol to yield acetyl CoA and OAA (ADP + Pi too of course)
|
|
|
Why can't acetyl CoA cross the mitochondrial membrane?
How does it circumvent this obstacle? |
mitochondrial membrane is impermeable to acetyl CoA. Must couple with OAA to make citrate to get to the cytosol where it can make some FA's!
|
|
|
Why are the bypass reactions which take place in order to get OAA into the mitochondria important?
|
They generate much of the NADPH needed to make FA's.
|
|
|
What are the bypass reactions needed to get OAA into the mitochondria
|
OAA reduced to malate by malate dehydrogenase
OAA + NADH + H+--> malate + NAD+ decarboxylation by NADP+-linked malate enzyme malate + NADP+ --> pyruvate + CO2 + NADPH recarboxylation by pyruvate carboxylase pyruvate + CO2 + ATP + H2O --> OAA + ADP + Pi + 2 H+ |
|
|
How many NADPH are generated from each acetyl CoA that is transferred from matrix to cytosol?
|
1 NADPH
|
|
|
How many acetyl CoA do you need to make palmitate?
How many NADPH does this number generate? Where do the remaining NADPH needed come from? |
8 acetyl CoA needed to make palmitate.
Therefore as the 8 acetyl CoA cross the mitochondrial membrane, 8 NADPH are generated. Penthose Phosphate pathway |
|
|
Ugh. Describe this bad boy
|
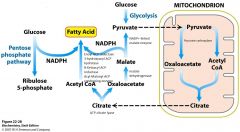
|
|
|
when is FA synthesis maximal?
|
when carbs and energy are plentiful and when fatty acids are scarce.
|
|
|
What is essential in controlling and regulating FA synthesis?
|
Acetyl CoA carboxylase catalyzes the production of malonyl CoA
|
|
|
How is acetyl CoA carboxylase regulated?
|
inhibited by AMP-dependent protein kinase (AMPK)
activated by Protein Phosphotase 2A (PP2A) |
|
|
How is AMPK like a fuel gauge?
|
AMP present activates AMPK and thus phosphorylates the carboxylase and inactivates the creation of malonyl CoA
The presence of ATP, inactivates AMPK and tells PP2A to go which dephosphorylates the carboxylase and it converts acetyl CoA to malonyl CoA. So when energy is abundant=go PP2A, go carboxylase when energy is scarce=go AMPK, stop carboxylase |
|
|
What does AMPK do?
What effect does this have on FA synthesis? |
phosphorylates Acetyl CoA carboxylase
halts FA synthesis |
|
|
What does PP2A do?
What impact does this have on FA synthesis? |
dephosphorylates Acetyl CoA carboxylase
initiates the starting of FA synthesis by carboxylating Acetyl CoA to malonyl CoA |
|
|
How do epi and glucagon impact ACC?
|
ACC stays phosphorylated
No FA synthesis |
|
|
How does insulin impact ACC?
|
dephosphorylates ACC, go FA synthesis
|
|
|
How does citrate impact ACC?
|
follow me here:
when ATP and acetyl CoA are abundant citrate is too means time is ripe for FA synthesis. converts the inactivated (phosphorylated) ACC into active filaments |
|
|
How does palmitoyl CoA and AMP impact ACC?
|
antagonizes citrate
causes the active filaments that citrate turned ACC-Pi into, into inactive filaments. |
|
|
What is the difference in the enzymes that start oxidation between mitochondrial matrix oxidation versus peroxisomal oxidation?
|
mito--acyl CoA dehydrogenase
periox--flavoprotein acyl CoA dehydrogenase the flavoprotein guy is the electron transferrer from O2 to H2O2 |

