![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
207 Cards in this Set
- Front
- Back
|
What are the "triangle" components of IMMUNITY?
|
1) Immune system
2) Nervous system 3) Endocrine system |
|
|
Define susceptibility.
|
Lack of resistance to a disease
|
|
|
What factors affect susceptibility of immunity?
|
1) General health
2) Nutrition 3) Age 4) Stress 5) Limited short term stress (exercise-->can stimulate immune response) |
|
|
What is resistance?
|
Ability to ward off disease
|
|
|
What is nonspecific resistance?
|
Generally present. Defenses against any pathogen (not directed toward any particular pathogen)
|
|
|
What is specific resistance?
|
Immunity, resistance to a SPECIFIC pathogen
|
|
|
What are the THREE characteristics of innate resistance/immunity?
|
1) Species
2) Race or strain 3) Gender |
|
|
How does the SPECIES characteristic play with regards to innate resistance/immunity?
|
Pathogens may only infect a limited range of species.
EX: Mumps: infects humans and not dogs or cats Anthrax: infects humans and cattle but not birds |
|
|
What are three characteristics within species that affect innate resistance/immunity?
|
1) Physiology (chemical make-up, temperature, etc)
2) Anatomy (chemical receptors) 3) Behavior/diet (what is consumed) |
|
|
With regards to Anthrax, what is key for its infection?
|
Temperature!
Affects humans around 37 C and birds around 41-45 C |
|
|
Which famous scientist exposed chickens to anthrax and then lowered their body temperature which lead to their infection with anthrax?
|
L. Pasteur
|
|
|
How does a specie's anatomy play a role in its innate resistance/immunity?
|
The anatomy of the specie can allow for infection or not.
EX: Plant disease--certain chemical receptors plant pathogens require do no exist in/on the human body) |
|
|
How does a specie's behavior/diet play a role in its innate resistance/immunity?
|
What we eat or our specific behavior can introduce infection.
EX: Fish tapeworm Diphyllobotrium latum. Ingested in raw fish. Any species that does not eat fish is unlikely to get this disease |
|
|
How does a specie's race or the particular strain play a role in the specie's innate resistance/immunity?
|
Certain races are more susceptible to certain strains and vice versa.
EX: Caucasians are more susceptible to diphtheria, influenza, and gonorrhea. Blacks and American Indians are more susceptible to TB |
|
|
How does a specie's gender play a role in its innate resistance/immunity?
|
Hormone levels effect the immune system
|
|
|
In trying to stay "modest" when listening to the breathing of his female patients, physician Rene Laennec used a tool he created which eventually became the what?
|
Stethoscope
|
|
|
What are the NINE mechanical/physical barriers against a pathogen?
|
1) Intact skin
2) Mucous membranes 3) Hairs of ears and nose 4) Ciliary/mucociliary escalator 5) Lacrimal apparatus 6) Salivary glands 7) Urine 8) Vaginal secretions 9) Reflexes |
|
|
How does intact skin protect against microbes?
|
Epidermis consists of tightly packed cells with Keratin, a protective protein--act as barrier to most microbes
|
|
|
How does mucous membranes protect against microbes?
|
Lines body cavities that open to exterior (digestive, urinary, respiratory, reproductive tracts). Epithelial layer of mucus membranes secretes mucus which acts as a trap and physical barrier--much less protective than skin
|
|
|
How does hairs of nose and ears protect against microbes?
|
Filter and trap microbes
|
|
|
How does ciliary/mucociliary escalator protect against microbes?
|
Microbes trapped in mucus are transported away from the lungs
|
|
|
How does the lacrimal apparatus protect against microbes?
|
manufactures and drains away tears, continual washing of the eyes
|
|
|
How does salivary glands protect against microbes?
|
Wash microbes from teeth and mucus membranes of the mouth
|
|
|
How does urine protect against microbes?
|
Flow of urine moves microorganisms out of the urinary tract
|
|
|
How does vaginal secretions protect against microbes?
|
Help remove microorganisms from the vagina
|
|
|
How do reflexes protect against microbes?
|
Coughing, sneezing, vomiting, diarrhea, and defecation all move and/or flush microbes out of the body
|
|
|
What are the FIVE chemical defenses against microbes?
|
1) Sebaceous glands
2) Sweat glands 3) The Beta defensins 4) Gastric juices 5) Transferrins |
|
|
How do sebaceous glands protect against microbes?
|
Secrete sebum--contains fatty acids--gives the skin a low pH (3-5)
|
|
|
How do sweat glands protect against microbes?
|
Produce perspiration--contains NaCl and lysozyme
|
|
|
How do Beta defensins protect against microbes?
|
Antimicrobial peptides implaicated in the resistance of epithelial surfaces to microbial colonization
|
|
|
How do gastric juices protect against microbes?
|
Contains HCl--low pH (1.2 - 3)
|
|
|
How do transferrins protect against microbes?
|
Iron-binding proteins in the blood. Limits available iron needed for microbial growth
|
|
|
What are some second line of defenses?
|
1) Natural killer cells (NK cells)
2) Phagocytosis 3)Inflammation/fever 4)Antimicrobial substances/various chemicals |
|
|
What do NK (natural killer) cells do?
|
Release granzymes (protein digesting enzymes) induce the target cell to undergo apoptosis (self destruction)
|
|
|
What does blood consist of?
|
Plasma and formed elements (cells and cell fragments suspended in plasma)
|
|
|
Which part of the blood contains antibodies and other elements not used in blood clotting?
|
Serum
|
|
|
Name the formed elements.
|
1) Erythrocytes (RBCs)
2) Leukocytes 3) Thrombocytes/platelets |
|
|
What are 2 types of Leukocytes?
|
1) Granulocytes
2) Agranulocytes |
|
|
What are the three different types of GRANULOCYTES?
|
1) Neutrophiles (polymorphonuclear leukocytes [PMNs])--normally 3-lobed
2) Basophils 3) Eosinophils |
|
|
What are the two types of AGRANULOCYTES?
|
1) Monocytes/Macrophages
2) Dendritic cells 2) Lymphocytes |
|
|
What is the function of RBCs?
|
Transport of O2 and CO2
|
|
|
What is the function of neutrophils (granulocyte)?
|
Phagocytosis--eat until they die. Neutrophils make up 60-70% of leukocytes
|
|
|
What is the function of basophils/mast cells?
|
Production of histamine (0.5-1% of leukocytes)
|
|
|
What is the function of eosinophils?
|
Production of toxic proteins against certain parasites; some phagocytosis (2-4% of leukocytes)
|
|
|
What are the functions of thrombocytes/platelets?
|
Blood clotting
|
|
|
What is the function of monocytes?
|
Phagocytosis--when they mature into into macrophages (3-8% of leukocytes)
|
|
|
What is the function of dendritic cells?
|
Derived from monocytes; phagocytosis and initiation of adaptive immune responses
|
|
|
What are the functions of lymphocytes?
|
NK cells. Destroy target cells by cytolysis and apoptosis. Destroy virus-infected cells and tumors. Involved in specific immunity (T-cells and B-cells specific response)
|
|
|
What are the two components of lymphocytes?
|
1) T-cells
2) B-cells |
|
|
What is the function of T-cells?
|
Cell-mediated immunity
|
|
|
What is the function of B-cells?
|
Descendants of B-cells (plasma cells) produce antibodies
|
|
|
What is a differential blood count?
|
Determines the relative percent of each WBC type. Many infections cause an increase or decrease in WBC numbers
|
|
|
What is leukocytosis?
|
An increase in total WBC count--indicates state of infection
|
|
|
What is leukopenia?
|
A decrease in WBC count-->anemia
|
|
|
What is neutrophilia?
|
An increase in neutrophile (phagocytes--most abundant in circulation) count.
|
|
|
What does a left band shift in neutrophil distribution mean?
|

Less than 10% bands = infection/inflammation --> band cells (young neutrophils). When neutrophils shift to younger cells (increasing in number of young neutrophils), it's a good indication of infection
|
|
|
What is neutropenia?
|
A decrease in neutrophil count
|
|
|
What is eosinophilia?
|
An increased in eosinophil count (e.g. allergies and parasitic infections)
|
|
|
What is lymphocytosis?
|
An increase in lymphocyte count
|
|
|
What is the mononuclear phagocytic/reticuloendotheilial system (RES)?
|
A system that consists of phagocytes which enter certain tissues and remain there to fight infection--fixed and wandering macrophages
|
|
|
What are fixed macrophages/histocytes?
|
Resident in certain tissues and ingest bacteria and debris as they flow past
|
|
|
Fixed macrophages/histocytes found in the liver are called?
|
Kupffer cells
|
|
|
Fixed macrophages found in the lungs are called?
|
Alveolar macrophages
|
|
|
Fixed macrophages found in the nervous system are called?
|
Microgial cells
|
|
|
Fixed microphages found on the skin are called?
|
Langerhan's cells
|
|
|
In addition to the lungs, skin, nervous system, and liver, where else can fixed macrophages found?
|
Spleen, lymph nodes, bone marrow, and peritoneal cavity
|
|
|
What are the phases of phagocytosis?
|
Chemotaxis, adherence, ingestion, and digestion
|
|
|
What is inflammatory response and its S/S?
|
A response to tissue damage by a combination of NONSPECIFIC defenses.
Characterized by: redness (RUBOR), pain (DOLOR), heat (CALOR), swelling (TUMOR/EDEMA) ***Swelling/edema is a classic characteristic of local inflammation*** |
|
|
What are the 4 functions of inflammation?
|
1) Destroy injurious agent and remove it
2) Confining/walling off the injurious agent and its by-products 3) Repair/replace damaged tissues 4) Stimulate/enhance immune response |
|
|
Which proteins are activated with increasing concentrations in response to injury?
|
Acute-phase proteins (complement, cytokine, fibrinogen, and kinins)
|
|
|
What is vasodilation?
|
Increasing diameter of blood vessels
|
|
|
Histamine, Kinins, Prostaglandins, and Leukotrienes all do what to blood vessels with regards to injury to tissues?
|
They are all vasodilators.
|
|
|
What are the S/S of vasodilation to injurious tissues?
|
Redness and heat, increased permeability/perfusion
|
|
|
Margination and emigration of WBCs to injurious tissue is known as what?
|
Diapedesis
|
|
|
Which short-lived leukocytes are first on scene of injurious tissue?
|
Neutrophils (PMNs)--eat until they die
|
|
|
Which longer lived leukocytes reach the scene injurious tissue after PMNs (neutrophils)?
|
Monocytes--hanger out until it's over
|
|
|
An increase flow of fluids from blood to tissue spaces is know as what?
|
Swelling/edema
|
|
|
The final stage of inflammation and begins during the active phase of inflammation is known as what?
|
Tissue repair
|
|
|
A systemic increase in body temperature is known as what?
|
Fever
|
|
|
What does Endogenous Pyrogens (Interlukin 1: IL-1) and alpha tumor necrosis factor do?
|
Heat-producing: increases over all body temperature = FEVER
|
|
|
Endogenous Pyrogens (Interlukin 1: IL-1) and alpha tumor necrosis factor are secreted by?
|
Monocytes and Macrophages
|
|
|
The body's termostat is the....?
|
Hypothalamus
|
|
|
Endogenous Pyrogens (Interlukin 1: IL-1) and alpha tumor necrosis factor act on which structure to increase body temperature?
|
Hypothalamus--releases prostaglandins to reset hypothalamic thermostat to higher temperature
|
|
|
What is it called when the skin becomes warm and the person begins to sweat (break a sweat) in the fever phase?
|
Crisis--an indication of falling body temperature (back to normal range). Happens with elimination of IL-1
|
|
|
What are the advantages of fever (increased body temperature)?
|
It can inhibit growth of some organisms and speeds up the body's chemical rxns
|
|
|
What is the complement system?
|
A non-specific response. A group of over 30 proteins found in the blood. Complements antigen antibody rxns--binds to immune complexes
|
|
|
What are the 4 functions of the complement system?
|
1) Cell lysis: membrane attach complex
2) WBC chemotaxis: attracts phagocytes 3) Opsinization or immune adherence: enhanced phagocytosis 4) Inflammation |
|
|
What are Interferons (IFNs)?
|
A class of antiviral proteins produced by certain animal cells (lymphocytes and macrophages) after viral stimulation
|
|
|
What are the 3 types of Interferons (IFNs)?
|
1) Alpha IFN
2) Beta IFN 3) Gamma IFN |
|
|
What is the function of Alpha and Beta IFNs (Interferons)?
|
Cause cells to produce antiviral proteins that inhibit viral replication (cell-to-cell signaling)
|
|
|
What is the function of Gamma IFNs (Interferons)?
|
Increases the activity of neutrophils and macrophages in phagocytizing bacteria (cell-to-cell signaling)
|
|
|
What are Toll-Like Receptors (TLRs)?
|
Transmembrane membrane proteins on immune cells that recognize and attach to molecules on pathogens--activate an immune response to those microbes.
TLRs recognize a variety of pathogen-associated molecular patterns (PAMPs) |
|
|
What are 2 types of specific host defenses of the immune system?
|
1) Humoral Immune system
2) Cellular Immune system |
|
|
What does the humoral immune system involve?
|
Specific antibodies in the blood and lymph--the body's humors. Produced by B-cells
|
|
|
What does the cellular immune system involve?
|
Involves T-cells. Do not produce antibodies but secrete cytokines
|
|
|
Where T-cells and B-cells originate from?
|
Bone marrow stem cells
|
|
|
Where to T-cells reside to mature and where do they migrate to after?
|
Thymus gland--circulate in lymphoid tissue
|
|
|
Where do B-cells reside to mature and where do they migrate to after?
|
Bursa of fabricius (birds), fetal liver, human Bone marrow--circulate in lymphoid tissue
|
|
|
What T-cells and B-cells respond to?
|
They are specialized lymphocytes respond to intracellular Antigens (Ags)
|
|
|
What are the 2 kinds of Immunity?
|
1) Acquired Immunity
2) Innate Immunity |
|
|
What is Acquired Immunity?
|
Resistance to infection due to activity of Antibodies
|
|
|
What are the 2 types of Acquired Immunity?
|
1) Naturally acquired ACTIVE Immunity--individual producing own antibodies
2) Naturally acquired PASSIVE Immunity--get preformed antibodies from someone or something else |
|
|
What are the 2 types of ACTIVE immunity?
|
1) Naturally Acquired Active Immunity
2) Artificially Acquired Active Immunity |
|
|
What is Naturally Acquired Active Immunity?
|
Stimulus: Contact with a live microbe by natural processes (infection, illness)
Response: Symptoms of disease or subclinical (no S/S) rxn--active production of specific antibodies to the pathogen Duration: Long term (months-years), sometimes life-long. Lots of exceptions: influenza |
|
|
What is Artificially Acquired Active Immunity?
|
Not a natural process (by IM/IV)
Stimulus: A) Vaccines/immunization (killed pathogens or their proteins alone. B) Attenuated/weakened live pathogens. C) Inactivated toxins (toxoids--an inactivated toxin) Response: Production of specific antibodies without developing symptoms of disease (or prodromal symptoms only) Duration: Variable (months-years-life time) |
|
|
What is Acquired Passive Immunity?
|
Immunity acquired through transfer of antibodies
|
|
|
What are the 2 types of Acquired Passive Immunity?
|
1) Naturally Acquired Passive Immunity
2) Artificially Acquired Passive Immunity |
|
|
What is Naturally Acquired Passive Immunity?
|
Mother to fetus through the placenta (placental transfer) or in colostrum/milk during nursing
Response: No immune response. Acquisition of antibody only Duration: Short term (few weeks-months): enough to protect until one's own immunity kicks in |
|
|
What is Artificially Acquired Passive Immunity?
|
Antibodies formed in one individual transferred/injected into another individual--immune serum/gamma globulin (preformed)
Response: No immune response, acquisition of antibody only Duration: Very short (2-3 weeks): venomous snake bites, tetanus, Hep. A, Diphtheria, Botulism |
|
|
What is the relationship between Antigens (Ag) and Antibodies (Ab)?
|
Specific immune response involves production of SPECIFIC antibodies (Ab) against specific antigens (Ag)
|
|
|
What is an IMMUNOGEN?
|
Any substance that when introduced into the body STIMULATES the production of SPECIFIC antibodies
|
|
|
What is an ANTIGEN?
|
Any substance that COMBINES with those specific antibodies
***The term antigen is often used to mean both an antigen and an immunogen*** |
|
|
What are the characteristics of an antigen/immunogen?
|
Foreign/non-self matter: microorganisms, toxins, foreign tissues
Chemically: complex chemicals (proteins or polysaccharide) |
|
|
What characteristics make a POOR antigen/immunogen?
|
1) Small molecules
2) Simple molecules 3) Large but repetitive molecules--no side groups |
|
|
What is a HAPTEN/PARTIAL antigen?
|
A molecule too small to stimulate antibody formation by itself. However, when it combines with a larger carrier molecule, usually a serum protein, the hapten and its carrier together form a conjugate that can stimulate an immune response (most be bound)
|
|
|
What is an antigenic determinant/epitope region on the pathogen?
|
Antibodies are not formed against a whole organism but specific regions or chemical groups on the surface of a pathogen
|
|
|
What is the definition of Antibodies/Immunoglobulins?
|
A protein produced by B lymphocytes in response to an immunogen/antigen and is capable of combing with that antigen
|
|
|
What are gamma globulins?
|
Serum fraction containing immunoglobulins (antibodies)--also called immune serum globulin
***artificially acquired passive*** ***obtained from gel electrophoresis*** |
|
|
Describe the generic structure of an antibody.
|
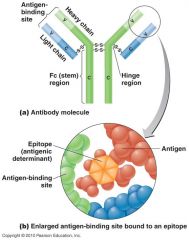
Two heavy chains and two light chains. Heavy and light chains both have a "C" or constant portion and a "V" variable portion
|
|
|
What is the V (variable) portion on antibodies?
|
Different for each kind of Ab and gives the Ab its specificity. Ag binding occurs at the V portion
|
|
|
What is unique about the C Portion of an antibody?
|
It is the constant for each class of Ab
|
|
|
Is the compliment binding site specific or non-specific?
|
It is very non-specific
|
|
|
The two identical sites that bind to epitopes on antibodies are called what?
|
FAB--Antigen binding fragment, two arms of the antibody
|
|
|
Where is the compliment binding site on the antibody?
|
Fc--Crystallizable fragment--the "trunk" of the antibody
|
|
|
What are the 5 classes of antibodies?
|
IgG
IgM IgA IgD IgE |
|
|
Describe the characteristics of an IgG Antibody
|
Monomer. 80% of serum antibody (most abundant Ab in serum)
Can cross the placenta: protects fetus & newborn Fixes complement. Enhance phagocytosis. Neutralizes toxins and viruses |
|
|
Describe the characteristics of an IgM Antibody
|
Pentamer. Fixes complement.
In blood, lymph, on B-cells. Aggulutinates microbes ***First Ab produced in response to infection*** |
|
|
Describe the characteristics of an IgA Antibody
|
Dimer. Main
***Ab in mucus secretions and breast milk*** Mucosal protection |
|
|
Describe the characteristics of an IgD Antibody
|
Monomer. In blood and lymph
***Receptor on B-cells*** |
|
|
Describe the characteristics of an IgE Antibody
|
Monomer. In blood
***On mast cells and basophils*** ***Allergic rxns; lysis of parasitic worms*** |
|
|
What is clonal selection?
|
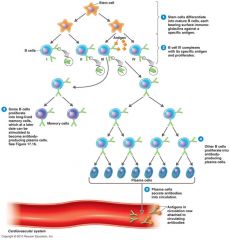
When an antigen is introduced, recognized by B-cells. B-cells differentiate into memory cells (for that specific antigen) and plasma cells (produce specific antibodies)
|
|
|
What is the antibody response (by B-cells)?
|
Primary response: contact with Ag for the first time = plasma cells (antibodies) and memory cells are formed
Secondary response/Anamnestic Response: Memory cells activated --> Plasma cells --> Abs formed quickly and in large numbers |
|
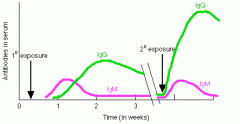
Explain this graph.
|
IgM appears first in response to the initial exposure. IgG follows and provides longer-term immunity. The second exposure to the same antigen stimulates the memory cells (formed at the time of initial exposure) to rapidly produce a large amount of antibody. The antibodies produced in response to this second exposure are mostly IgG.
|
|
|
Review Adaptive Immunity chart.
|
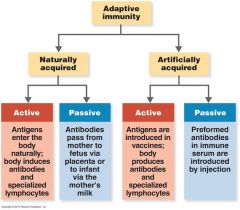
Review Adaptive Immunity chart.
|
|
|
What are the results of Ag-Ab binding?
|
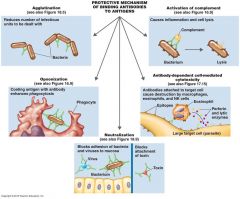
The binding of Ag-Ab to form Ag-Ab complexes tags foreign cells and molecules for destruction by phagocytes and complement
|
|
|
What is cell mediated immunity and is it specific or non-specific?
|
Involves interaction of macrophages and specific T lymphocytes (T-cells). A specific host defense
|
|
|
What are APCs (antigen-presenting cells)?
|
A macrophage, dendritic cell or B-cell that engulfs an antigen and presents fragments to the T-cells
|
|
|
What are dendritic cells?
|
A type of antigen-presenting cell characterized by long finger like extensions; found in lymphatic tissue and skin
|
|
|
Which cells help facilitate contact of antigens with the immune system?
|
M cells (microfold)
|
|
|
Where are M cells (microfold) found?
|
Within Peyer's patches in the intestinal wall
|
|
|
What do T-cells differentiate into when stimulated by an Ag?
|
Effector T-cells
|
|
|
What can some Effector T-cells become?
|
Memory cells
|
|
|
What do T-cells produce?
|
Cytokines--small proteins released from human cells that regulate the immune response, directly or indirectly many induce fever, pain or T-cell proliferation
|
|
|
What are the 3 type of T-cells?
|
1) Helper T-cells (CD4, TH)
2) TH1: Activate cells related to cell-mediated immunity 3) TH2: Activate B-cells to produce IgM and IgE |
|
|
What do CYTOTOXIC T-cells (CD8, TC) do?
|
Destroy target cells with PERFORIN and GRANZYMES (self-destruction)
|
|
|
This time of T-cell is associated with allergic rxn, transplant rejection, TB skin test, and contact dermatitis.
|
TD cells (D is for delayed)
|
|
|
Which T-cell turns of immune response when Ag is no longer present?
|
Regulatory (TR) T-cells [formerly TS--Suppressor T-cells]
|
|
|
What are activated macrophages?
|
Nonspecific cells. Macrophages stimulated by ingesting Ag or by cytokines
|
|
|
What are Natural Killer cells?
|
Lymphocytes that destroy virus-infected cells and tumors.
|
|
|
What is the difference between T-dependent Antigens and T-independent Antigens?
|
T-dependent antigens requires contact with T-cells to stimulate the formation of antibodies.
T-independent antigens does not require contact with T-cells to stimulate the formation of antibodies |
|
|
What is Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)?
|
The killing of antibody-coated cells by natural killer cells and leukocytes
|
|
|
What is meant by SELF-TOLERANCE?
|
The immune system does not normally attack self tissues or compounds
|
|
|
What is CLONAL DELETION?
|
During embryonic development lymphocytes with antigen receptors for molecules present in the are destroyed
|
|
|
What are Major Histocompatibility Complex (MHC) [same as Human Leukocyte Antigen Complex (HLA)]?
|
Glycoproteins in plasma membrane that enable the immune system to distinguish self from non-self
|
|
|
What are the classes of Major Histocompatibility Complex?
|
Class I MHC: on all nucleated cells (designation as "friend/self")
Class II MHC: On macrophages and B-cells |
|
|
Anaphylactic Rxn/Anaphylaxis invovles which antibodies and where does those antibodies bind?
|
Involves IgE antibodies which bind to Basophils/Mast cells
|
|
|
What happens during the 1st exposure to an allergen?
|
IgE is formed and binds to Mast cells by the Fc portion (No symptoms at this point)
|
|
|
What happens during the 2nd exposure the (same) allergen?
|
Histamine, Leukotriene, Prostaglandins released
1) Dilates blood vessels 2) Increased permeability of capillary vessels 3) Contraction of smooth muscles 4) Increased mucus secretion 5) Increased secretion of HCl in stomach |
|
|
How many types of hypersensitivities to allergens are there?
|
4:
Type I (Anaphylactic)--Immediate (10-20 mins) Type II (Cytotoxic) Type III (Immune Complex) Type IV (Delayed Cell-Mediated) |
|
|
Two major types of Type I (Anaphylactic) rxns are? Which one is more common?
|
Systemic Anaphylaxis and Localized anaphylaxis/Atopic disease (more common of the two)
|
|
|
What is systemic anaphylaxis?
|
Severe, sometimes fatal. Develops rapidly after antigen/allergen is introduced to a sensitized (IgE antibodies to particular antigen)
EX: Bee sting: shock and asphyxia |
|
|
What are some S/S of systemic anaphylaxis?
|
Flushing of skin (vasodilation)
SOB (smooth muscle contraction/constriction) Shock Constriction of smooth muscle in the lungs Asphyxia: death due to respiratory failure |
|
|
What is a treatment for systemic anaphylaxis?
|
Adrenaline/Epinephrine
|
|
|
What are some examples of localized anaphylaxis/Atopic disease?
|
Hayfever, asthma, hives antigens/allergens: plant pollen, fungal spores, dust, animal dander, dust mites, foods
|
|
|
Which antibodies does a localized anaphylaxis/atopic disease involved in hayfever?
|
IgE. Allergic rxn of the upper respiratory (atopic)
|
|
|
What are the S/S of a localized anaphylaxis/atopic disease such as hayfever?
|
Itchy, tearing eyes, congested nasal passages, runny nose and sneezing.
|
|
|
What is the treatment for a localized anaphylaxis/atopic disease such as hayfever?
|
Antihistamine
|
|
|
What is asthma?
|
A localized anaphylaxis/atopic disease. Allergic rxn of the lower respiratory system.
|
|
|
What are the S/S of asthma?
|
Wheezing and SOB
|
|
|
What is treatment for asthma?
|
Adrenaline, Epinephrine (Leukotrienes and NOT Histamine)
|
|
|
What is DESENSITIZATION?
|
Carefully injecting small, repeated doses of the allergen into the skin. Patient produces IgG antibody rather than IgE.
IgG acts as "blocking" Ab. Prevents binding of allergen to IgE on Mast cells --> No release of histamine --> no symptoms |
|
|
Which antibodies are involved in Type II (Cytotoxic) Rxns?
|
IgG or IgM antibodies and complement.
Complement activation causes cell lysis or damage by macrophages. EX: Transfusion rxns and Rh incompatibility |
|
|
Study the ABO Blood Groups.
|
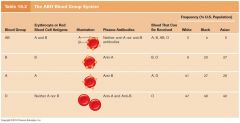
Study the ABO Blood Groups.
|
|
|
Antigens on surface of RBCs are made of what?
|
Carbohydrates
|
|
|
Incompatible blood types will cause clumping of RBCs, which is also known as?
|
Hemagglutination
|
|
|
What is Rh (D Ag in humans) Incompatibility and what does it cause?
|
Results from Rh negative mother expose to Rh positive cells by prior birth or transfusion (Sensitized).
1) Produce anti-Rh antibodies (IgG) which can cross the placenta 2) Reacts with fetal RBCs --> lysis of RBCs. Results in release of toxins (Bilirubin) and anemia Causes erythroblastosis fetalis/hemolytic disease of the newborn (HDN) |
|
|
What is treatment for Rh Incompatibility?
|
Exchange transfusion: removal of fetal Rh+ cells and transfusion with Rh- blood.
|
|
|
What is a way to prevent Rh Incompatibility?
|
RhoGam shot: passive immunization of Rh- mother with anti-Rh antibodies. Blocking Ab coats Rh+ cells and prevents sensitization
|
|
|
What is Drug-induced Thrombocytopenic Purpura?
|
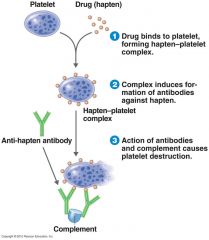
Molecules of a drug such as quinine accumulate on the surface of a platelet and stimulate an immune response that destroys the platelet
|
|
|
What is Type III (Immune Complex) Hypersensitivity?
|
Excess IMMUNE COMPLEXES are deposited in tissues --> localized inflammation and tissue damage.
Involves antibodies against soluble antigens circulating the serum. |
|
|
What are some examples of Type III (Immune Complex) rxns?
|
Non self Ag: Serum sickness
Self Ag: Autoimmune disease Rheumatoid Arthritis Systemic Lupus Erythematosus Glomeruloephritis |
|
|
What are Type IV (Delayed) Hypersensitivity rxns?
|
Rxns involve cell-mediated immune responses and are caused mainly by T-cells. Not apparent for a day or more.
|
|
|
What is an example of Type IV (Delayed) Hypersentivity rxn?
|
Contact Dermatitis: T-cell mediated response
Allergen binds to some of your own cells (EX: Poison Oak, Poison Ivy) T-cells infiltrate tissue --> Cytokines --> activate inflammatory cells --> destroy target cells |
|
|
What are Autoimmune Disorders?
|
Production of autoantibodies (Antibodies against ones own tissues or chemical substances) or self-reactive T-cells
|
|
|
Who is the guy associated with the term Horror Autotoxicus?
|
Paul Ehrlich ("Magic Bullet")
The need to not react to self. Deliberately attempted to immunize animals against their own tissues |
|
|
Give examples of Autoimmune Disease with the 4 types of hypersensitivities.
|
Type I: Due to antibodies against pathogens
Type II: Antibodies react with cell-surface antigens Type III (Immune Complex): IgM, IgG, complement immune complexes deposit in tissues Type IV: Mediated by T-cells |
|
|
What type of autoimmune hypersensitivity is Rheumatic Fever and Acute Glomerulonephritis?
|
Type I
Follows infection with Group A Streptococci (GAS)--cross-reactive antibody can bind to heart, cartilage, muscle, and kidney tissues |
|
|
What type of autoimmune hypersensitivity is Graves Disease?
|
Type II
Attachment of AutoAbs to receptors for Thyroid-Stimulating Hormones (TSH) on follicle cells of the thyroid gland. Stimulation of follicle cells --> overproduction of thyroxin (increases metabolism). This bypasses normal feedback control |
|
|
What is Hyperthyroidism?
|
As known as Goiter. Increased metabolic rate, nervousness, tremor, difficulty concentrating, heat intolerance, diarrhea, and palpitations.
Goiter is enlargement of Thyroid due to Graves Disease--TSH on follicle cells of thyroid are stimulated to overproduce thyroxin--autoimmune hypersensitivity of Type II |
|
|
What type of autoimmune hypersensitivity is Myasthenia Gravis?
|
Type II
AtuoAbs bind to acetylcholine receptors at neuromuscular junctions --> causes degradation of receptors --> muscle weakness and fatigue --> loss of muscle function --> paralysis --> respiratory failure and death Smooth muscle unable to contract. |
|
|
What type of autoimmune hypersensitivity is Systemic Lupus Erythematosis (SLE or Lupus)?
|
Type III
AutoAbs produced against a variety of tissues and DNA. Most involved: Kidneys, bone marrow, skin, nervous system, joints muscles heart GI tract |
|
|
What are some S/S of Systemic Lupus Erythematosis (SLE or Lupus)?
|
Fever, skin rashes, arthritis, effusions (fluid build up) and inflammation in pleural, pericardial, peritoneal cavities and CNS.
Can cause a life-threatening, progressive, immune complex-mediated glomerulonephritis (particularly against one's own DNA). 90% of case are in females of childbearing age |
|
|
What is Klinefelter's Syndrome?
|
A rare Systemic Lupus Erythematosis (SLE or Lupus) found in males.
Affected individuals have an extra X chromosome: XXY |
|
|
What type of autoimmune hypersensitivity is Rheumatoid Arthritis?
|
Type III
Associated with advanced aged (80% of population) Characterized by chronic inflammation of the joints AutoAbs (Rheumatoid factor) form immune complexes with IgG --> bind to synovial membrane of joints --> activates complement --> scar tissue and joint destruction |
|
|
What type of autoimmune hypersensitivity is Multiple Sclerosis (MS)?
|
Type IV
Damage to myelin sheath (demyelination) of CNS (brain and spinal cord) T-cells attack myelin --> decreased ability to conduct nerve impulses --> muscular weakness, tremors, difficulties in speech and vision --> paralysis |
|
|
What type of autoimmune hypersensitivity is Hashimoto's/Autoimmune Thyroditis?
|
Type IV
T-cells destroy the follicle cells that produce Thyroxin --> HYPOTHYROIDISM: malaise, lethargy, pain, fever, and increased risk of PYOGENIC (pus) infection. Opposite of Graves Disease. Treatable with given synthetic Thyroxin |
|
|
What type of autoimmune hypersensitivity is Diabetes Mellitus?
|
Type IV
Type I DM (Juvenile-onset/Insulin Dependent): affected individuals have to given themselves injections of insulin T-cells attack ISLET CELLS (B-cells) of the pancreas --> inflammatory rxn --> cell lysis --> decreased insulin secretion |
|
|
Study insulin cycle.
|
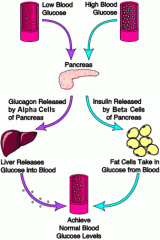
Study insulin cycle.
|
|
|
What type of autoimmune hypersensitivity is Sjogren's Syndrome (pronounced SHOW-grins)?
|
Type IV
Second most common disease after Rheumatoid Arthritis. Immune cells attack moisture producing/secreting glands (EX: tears) |
|
|
What Acquired Immune Deficiency Syndrome (AIDS)?
|
AIDS is caused by HIV (Human immunodeficiency virus [Retroviridae]). AIDS is not AUTOIMMUNE.
This virus is envelope with transmembrane spikes (glycoprotein) GP120 spikes enable HIV to attach to host cell with the CD4 receptor. CD4 receptors are found on T-cells, marcophages dendritic cells, and monocytes. Upon enering a cell, viral RNA is transcribed into DNA by the enzyme REVERSE TRANSCRIPTASE Viral DNA becomes integrated into the host cell chromosome. Integrated DNA may be ACTIVE or LATENT (can be delayed as long as 15+ years). |
|
|
How many clinical categories of HIV infection are there?
|
Three: A, B, C (least to worst)
|
|
|
Describe characteristics of Category A of HIV infection.
|
Category A:
Asymptomatic or lymphadenopathy (swollen lymph nodes). CD4 count 500/mm3 or above |
|
|
Describe characteristics of Category B of HIV infection.
|
Category B:
Various symptoms and persistent infections (oral candidiasis--Thrush) CD4 count between 200-499/mm3 |
|
|
Describe the characteristics of Category C of HIV infection.
|
Category C:
Clinical AIDS. Various opportunistic infections. Less than 14% of T-cells present are CD4 cells CD4 count BELOW 200/mm3 |
|
|
How is HIV transmitted?
|
Direct contact with body fluids:
Blood, Semen, Saliva (gum bleeds) [most common to least] |
|
|
What are some treatments for HIV?
|
Nucleoside Analogs (bogus/fake bases): AZT, ddl, ddC
EX: AZT inhibits reverse transcriptase as it synthesizes DNA (1st punch). It resembles thymidine but lack the correct attachment for the next nucleotide. Protease inhibitors: ritonavir and indinavir (2nd punch)--block an enzyme needed to assemble the virus The combination of a nucleoside analog and protease inhibitor is the best method for retarding an HIV infection right now (more synergistic) |
|
|
What are some problems associated with developing a vaccine for HIV/AIDS?
|
1) No ideal animal host
2) Virus targets the immune system itself 3) Mutation rate of HIV is high 4) Needs both antibody and killer T-cell response Effective vaccine will also have to stimulate a mucosal immune response --> IgA cross-reactive antibodies. |

