![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
236 Cards in this Set
- Front
- Back
|
What is intracellular osmoregulation? |
The active regulation that guarantees the absence of pressure gradients across the plasma membranes. It is also called cell volume regulation. |
|
|
Extracellular osmoregulation |
active, homeostatic regulation that maintains the osmotic concentrations in the body fluids, even if the osmotic concentrations of the environment change. |
|
|
osmoregulation? |
maintains the water and electrolyte content of an animal body. Because the most abundant electrolytes in body fluids and aquatic environments is Na and Cl (or NaCl), the mechanism of osmoregulation address usually active and passive movements of water and NaCl across cell membranes and the body surface. |
|
|
Where did al animals first live? |
the sea (marine) |
|
|
Describe the marine environment |
no osmotic gradients. little or no water movement. no energy investment into osmoregulation. body surface with high permeability. Some ion regulation. |
|
|
What are osmoconformers? |
animals in which the osmotic concentration of body fluids is uniform with the environment. However, solute make up can be different- ionoregulators. |
|
|
Describe the terrestrial environment |
humid places near water, in holes or under stones reduces evaporation. Decreased water permeability of body surfaces. Some adaptions to save water. |
|
|
adaption for animals from the sea to brackish water. (in between fresh water and salt water, where rivers meet the sea.) Describe brackish water environment |
-cell volume regulation. No osmotic gradient across body surface. intracellular osmoregulation to adapt cells. body surface with high permeability. Some cellular ion regulation. |
|
|
the adaption from less to more diluted brackfish water. |
body fluid hyperosmotic. passive water uptake, ion loss. hyperregulator |
|
|
adaptions for brackish to freshwater |
body fluid very hyperosmotic.active NaCl absorbtionand urine production. body surface with low permeability. Some cellular ion regulation. |
|
|
from freshwater to land adaptions |
inherited a low blood osmolarity. inherited a low body-surface permeability. inherited some transport mechanisms for NaCl and water regulation. ancestors came from freshwater |
|
|
fresh water back to sea adaptions |
-inherited low blood osmolarity, resulting n a reversed gradient in the sea indicating ancestors from freshwater. water loss compensated by drinking. salt gain is compensated by active NaCl secretion. hypo regulator |
|
|
percent vertebrate body volumes |
plasma=7 percent interstitial volume= 29 extracellular fluid= 36 intracellular fluid= 64 water content=60-80 percent total weight |
|
|
Interstitial space |
fluid the cells are bathed in but does not circulate |
|
|
Transcellular fluid |
CSF, digestive fluids, mucous |
|
|
plasma |
found in the circulation and bathes red blood cells. big part in overall volume and solute regulation. Small component of ECF. Directly impacted by the environment |
|
|
Rle of plasma volume |
supply nutrients and oxygen to all living cells and tissues. Volume homeostasis- water movement hypovolaemia (dehydration)- reduces P. volume hypervolaemia (hydration)- increased P. volume |
|
|
While the intracellular and extracellular environments differ, mechanisms work to maintain what |
constant osmotic pressure |
|
|
organic molecules are useful for cell volume regulation for what 2 important reasons? |
1. cell volume is not disrupted in the face of changing external osmolality. 2.Intracellular inorganic ion concentrations is not disrupted. |
|
|
Why are most animals similar in the amount of inorganic ions in the intracellular environment? |
these levels are required for optimal cellular function. Organic solutes are less disruptive to cellular functions. |
|
|
Describe homeostatic regulation of the "internal milieu" as a cornerstone of vertebrate success |
Solute and water balance in the ICF is dependent on the solute andwater balance of the ECF.There is free and regulated movement of ions, solute and wateracross cell membranes.The internal milieu must be maintained around a set point for optimalcellular function.The ECF acts as a “buffer” to the intracellular environment
|
|
|
challenges of regulation of extracellular fluids |
a) Body surface area vs. body volume
b) Osmotic gradient to the environment c) Permeability of the epithelium |
|
|
functions of the gill or lung epithelia or challenge |
must be thin, usually single layer for gas exchange. must not allow uncontrolled solute or ion fluxes. |
|
|
Tight junctions |
branching network of sealing strands that surround epithelia cells. |
|
|
3 major functions of the tight junctions. |
A) Hold the cells together
B) They block the movement of integral membrane proteinsbetween the apical (outside) and basolateral (“blood side”)surfaces of the cell -> polarized cell with distinguished functionsfor the apical and basolateral membrane. C) Prevent the passage of molecules and ions through the spacebetween cells (intercellular/paracellular pathway). So materialsmust actually enter the cells in order to pass through the tissue.This pathway (transcellular pathway) provides control over whatsubstances are allowed through. |
|
|
An organ for osmotic and ion regulation |
contractile vacuole- found in protozoans and poriferans. H-cells in nematodes, protonephridia, metanephridia, mollusc kidney, crustacean anntenal glands and gills |
|
|
Describe the contractile vacuole |
in sea water, the activity/frequency of the CV is low, but increases with dilution of the external medium. The volume of protozoa increases with diluted media, but is stabilized by the activity of the CV. |
|
|
What inhibitor makes the CV lose function and causes the cell to burst |
cyanide-inhibitor of cytochrome c oxidase |
|
|
what is located in the membrane of the CV |
aquaporins and V-ATPase |
|
|
CV in freshwater amoebe expel what |
a diluted fluid rich in sodium and poor in potassium. Hyposmotic fluid in the vacuole. |
|
|
cells of the H-cells in nematodes |
duct cell, excretory cell, gland cells, pore cells. |
|
|
experiment technique for H-cells in nematodes |
Selective ion electrode technique |
|
|
what are protonephridia |
Excretory organs of Plathyhelminths, Nemertines, Rotifers, Cestodes and otheraschelminthes. Also in larvae of molluscs, annelids, etc.
|
|
|
Descrive protonephridia |
blind ending channels with cilia in cap cell (filtration). Tubule cells reabsorb. Urine hypoosmotic. |
|
|
Describe the mechanism of protonephridia |
Cap cell has cilia. Cap and tube cells form a filter allowing for the passage of small ions. Cilia creating pressure and vacuum sucking in fluids.No proteins or large molecules can pass. (selective) May excrete glucose and good molecules. After the first filtration, there are mechanisms to regain these good molecules. |
|
|
what are metanephridia |
excretory organs of adult annelids (SW to FW polychaetes, earthworms, leeches) |
|
|
describe metanephridia |
liquid take up occurs in previous coelom. Ciliated funnel (nephrostome) moves coelom fluid into nephridium. Secretion and reabsorption along the tubule. |
|
|
Describe the mollusc kidneys |
filtration across the wall of the heart into the pericard. Secretion and reabsorption along the urinary duct. Marine molluscs produce urine that is isoosmotic to the hemolymph. FW molluscs produce hypoosmotic urine. Terrestrial molluscs produce urine with hardly any water content, using uric acid as a final product of protein/amino acid catabolism. |
|
|
Describe the crustacean antennal glands as a method of excretion |
Filtration to produce a primary urine. Secretion and reabsorption along the urinary duct to produce the final urine. (mainly for water volume regulation and divalent cations.) Marine produce isoosmotic urine. Fw hypoosmotic. Terrestrial pass their urine over the gills where water and salt are reabsorbed. |
|
|
Describe crustacean gills as a method of excretion |
organ of osmotic and ion regulation. Hyperregulators are terrestrial crustaceans that absorb NaCl and calcium across the gill epithelium. Hypregulators actively secrete NaCl across the gills. |
|
|
Describe fish gills for regulation |
organs of osmotic and ion regulation. hyperregulators (FW fish) absorb NaCl and calcium across the gill epithelium. Hypregulators (SW fish) actively secrete NaCl across the gills. |
|
|
Describe the malpighian tubules as a method of excretion |
excretory organ of land living insects. |
|
|
what transporters are found in the mosquito malphigian tubule |
H+/ATPase and Na+/K+/ATPase |
|
|
what can you use for an excretion experiment for tissues, especially for the malpighian tubules? |
Ramsay assay- looking for fluid secretion from a tissue using ringer solutuion and oil |
|
|
site of osmoregulation is mosquito larva |
anal papillae |
|
|
what happens to the papillae with decreasing osmoregulatory demands |
their papillae will shrink |
|
|
technique for determining ion concentration gradients or fluxes |
ion selective micro Electrode technique |
|
|
Transporters found in the papillae |
H+/ATPase and Na+/K+/ATPase |
|
|
Where is the site for osmoregulation in the Eurythoe complanata (fire worm) polychaete? |
A band of cilia encircles each finger of the gill- ventilation? gill has less cuticle more SA seen in the gill small distance from the blood vessel to the surface -well vasculated genes for ion transport and gas exchange higher expressed in the gills -Must be the gills! |
|
|
Function of the vertebrate kidney |
-Regulates ion balance -Regulates extracellular fluid volume -Nitrogenous waste excretion (mammals, not really fish) -Acid Base balance -Filtration begins at the glomerulus |
|
|
Where does pressure filtration occur in the vertebrates. what produces the pressure? |
At the bowmans capsule Colloid osmotic pressure caused by proteins (larger molecules) that cannot diffuse through the membrane |
|
|
What creates the filter in the bowmans capsule? (Filter between the blood vessels and the capsule) |
Combination of pores in the endothelia and pedicels of the podocytes create a filter permeable for water, ions and small molecules. (glucose, urea, amino acids) |
|
|
Describe the mechanisms in the loop of henle |
-lower osmotic concentration in the descending limb -conc become higher is decending from h20 leaving. -descending limb is impermeable to NaCl -lower ascending limb is permeable to NaCl and at the top it is actively excreted. -conc gets lower in the ascending -ascending is impermaeble to H20 so NaCl and H20 are kept in the body. -increasing concentration from the cortex to medulla (top to bottom) |
|
|
Describe the renal excretion of urea process |
1) Active reabsorption of NaCl in the thick ascending limb of the loop of henle and distal tube
2) leads to passive h20 reabsorption- concentrated filtrate with high urea conc entering the collecting duct 3) medullary collecting duct becomes permeable for urea- urea leaks out along gradient 4) urea increases interstitium osmolality drawing more H20 from the descending loop, very high NaCl conc at bottom of the loop 5) NaCl leaks out in the thin ascending limb along gradient |
|
|
What does final urea secretion depend on |
H20 reabsorption in the collecting collecting duct |
|
|
diuresis and anti-diuresis leads to what |
high water excretion and low water excretion |
|
|
what can regulate diuresis |
Vasopressin |
|
|
High vasopressin does what |
insertion of aquaporins and urea transporter in the apical membrane of the CD |
|
|
General principle of kidney structure (vertebrate, mollusc, proto-metanephridia, Malpghian tubule, antenall glands, excretory cell.) |
-Internal organs (no exchange with environment) - No uptake of molecules from environment - No gas exchange -Excretion only ( waste and ions or water) -volume regulation - First secretion/ ultrafiltration: unspecific then re absorption of valuable molecules |
|
|
General principle of gill structure |
-External organ (exchange with environment) - Well vasculated - Well ventilated - Uptake of molecules from environment -Excretion of ions and wastes (ammonia, co2) -Gas exchange -Increase of SA (transport area) -mitochondria rich (energy for transport) |
|
|
What kind of life cycle do the european eel and the chinese mitten crab have |
catadromous |
|
|
LF of salmon? |
Anadromous |
|
|
Do freshwater fish drink? |
no |
|
|
do SW fish drink? |
yes |
|
|
describe the FW osmoregulation strategy for an adapted teleost fish |
-Water entry over the skin and gills - ions ingested in food -Active uptake of ions over gills -some loss of ions via feces - Production of dilute urine, leading to high water loss and some ion loss. FW osmolality= 5mOsm/kg Plasma osmolality= 300mOsm/kg |
|
|
describe the SW osmoregulation strategy for an adapted teleost fish |
-Water loss over the skin and gills -drinking of SW - active excretion of monovalent ions by MR cells in the gill - Ions (mostly divalant) lost in feces - divalent ions and some water lost in scant urine. |
|
|
What are the functions of gills in different salinities |
-FW gills actively take up ions -SW gills actively remove ions -all this carried out by MRC's mainly |
|
|
What are the major players for transport |
Na+/K+/ATPase, Na+/K+/Cl- Freshwater: H+/ATPase , Carbonic Anhydrase , HCO3-/Cl- exchanger, Na+ channels, NHE (Na+/H+ exchanger) and NBC (sodium,bicarbonate transporter) |
|
|
describe Na/K/ATPase transporter |
-usually on the basolateral membrane -electrogenic transport of cations -generates the negative membrane potential -workhorse of cell -needs ATP-mitochondria -partners with k+ channels |
|
|
inhibitor of k+ channels? |
Ba2+ and lidocain |
|
|
describe the sodium potassium chloride cotransporter |
-electroneutral transport of Na,K and 2CL- ions -secondary active, energized by Na/K/ATPase-low intracellular Na concentration -depending on whether NaCl is absorbed or secreted, localized on the basolateral or apical membrane -works with chloride channels |
|
|
inhibitor of sodium potassium chloride cotransporter |
Bumetanide, Furosemide |
|
|
inhibitor of chloride channels |
DPC, NPPB |
|
|
Describe the salt secreting cell |
basolateral: Na/K/Cl cotransporter (pointing inwards), Na/K/ATPase, potassium channel apical: chloride channel Intracellular Cl- conc. and negative membrane potential drives Cl- out of the cell . Transepithelial potential promotes paracellular Na+ diffusion |
|
|
Describe the salt absorbing cell |
basolateral: chloride channel, Na/K/ATPase, potassium channel. apical: Na/K/Cl (pointing inwards), potassium channel (pointing outwards) Negative transmembrane potential and intracellular Cl concentration drives Cl out of the cell into plasma, and Na+ follows |
|
|
describe the H+ ATPase |
-electrogenic H+ excretion -generates an even more negative cell potential - partners with carbonic anhydrase and bicarbonate/Cl- exchanger |
|
|
inhibitor of H+ ATPase |
Bafilomycin, Concanamycin |
|
|
inhibitor of carbonic anhydrase |
Acetazolamide |
|
|
function of carbonic acid |
provide H+ and bicarbonate (from H2O and CO2) |
|
|
describe the bicarbonate cl- exchanger |
-electroneutral exchange -driven by Cl- and bicarbonate gradient |
|
|
inhibitor for bicarbonate Cl- exchanger |
DIDS |
|
|
describe the sodium channels |
-electrogenic transport -usually in the apical of FW species -driven by Na+ gradient and transmembrane potential |
|
|
inhibitor of sodium channels |
low concentration of Amiloride, Phenamil |
|
|
describe NHE |
-electroneutral transport -driven by Na+ gradient and pH |
|
|
inhibitor of NHE |
high concentration of Amiloride, EIPA |
|
|
describe NBC |
-electrogenic cotransport (one direction) of 2Na+ and 3HCO3- -driven by bicarbonate and/or sodium gradient |
|
|
describe the simplified FW cell |
basolateral: chloride transporter, Na/K/ATPase, potassium channel cytosol: carbonic anhydrase forming bicarbonate and protons apical: sodium channel, bicarbonate chloride exchanger, H+ATPase, NHE |
|
|
Reason and function of the fresh water cell model |
H+ATPase generates positive potential over apical membrane, driving in sodium ions. Fresh water animals have very tight epithelium, minimal paracellular diffusion |
|
|
characteristics of fish MRC's |
-large ovoid shape - high density of mitochondria -polarized (difference in apical and baso.) - also present in FW although they are characterized by a different form and function -secreting a lot of chloride in SW |
|
|
features of sea water MRC |
-large with deep apical pits -extensive baso. tubular system - sub-apical tubulovesicular system |
|
|
what do SW MRC's usually exist in |
multicellular complexes with accessory cells and other MRC's |
|
|
MRC's and AC's often share what? |
the same apical pit and the junction between AC's and MRC's is often shallow. Therefore leaky to ion movement |
|
|
features of freshwater MRC |
-smaller cell, shallow pit - less extensive baso. tubular system - sub apical tubulovesicular system |
|
|
do the FW MRC's exist in complexes? |
no |
|
|
describe the junctions of FW MRC's with pavement cells |
multi stranded and tight |
|
|
two different types of mrc's in the FW gills |
PNA- (former alpha) and PNA+ (former beta) MRC's |
|
|
describe PNA- or alpha mrc's |
-osmium staining is light -smooth apical membrane - well developed tubular system -long and thin - found at the base of the lamellae |
|
|
describe PNA+ or beta mrc's |
-osmium staining is dark -complex apical membrane projections - less well developed tubular system -large ovoid shape - found in intralamellar region |
|
|
cell model for FW PNA- MRC |
basolateral: Na/K/ATPase, NBC (towards blood) cytosol: carbonic anhydrase apical: H/ATPase, sodium channel, NHE |
|
|
cell model for FW PNA+ MRC |
basolateral: chloride channel, H/ATPasae, N/K/ATPase cytosol: carbonic anhydrase apical: Bicarbonate chloride exchanger, Na/K/Cl- |
|
|
are most crustaceans osmoconformers or regulators |
osmoconformers- not a lot of NaCl transport in the gills |
|
|
where does the green shore crab live |
intertidal zone or brackish habitats -can tolerate low salinities |
|
|
what does C. maenas/ green shore crab do in brackish water |
hyperregulater. Due to osmoregulating capabilities they are a great invader. |
|
|
where is the site of osmoregulation in crabs |
the posterior gills (high activity of Na/K/ATPase with decreasing salinity) |
|
|
experiment to measure osmoregulation in crab gills (or other tissues in organisms too) |
transepithelial potentail difference (PDte) decreases with increasing environmental salinity |
|
|
what does the transepithelial potential difference depend on? |
Na/K/ATPase |
|
|
how can you investigate the split gill lamellae of C. maenas for example? |
-using chamber - employing specific inhibitors for transporters and measuring changes in Na, Cl- fluxes - tracer ions for Na and Cl -electrical parameters: Rte, Isc, PDte |
|
|
cell transport model of a hyper-regulating crab |
cuticle: Na and Cl going through basolateral: chloride channel, potassium channel, Na/K/ATPase cytosol: Carbonic anhydrase apical: bicarb/chloride exchanger, NHE, Na/K/Cl, potassium channel, All creates negative Isc |
|
|
is the Chinese mitten crab cata or anadromic? |
catadromic |
|
|
is the chinise mitten crab a conformer? How do they do it? |
no they are an osmoregulator (hypo and hyper, euryhaline with tight epithelium. Upregulates NKA and H+ATPase for osmoregulating. They use the FW NaCl uptake model |
|
|
describe the true freshwater crab |
-entire life in FW -very tight epithelium -asymmetrical osmoregulatory gill lamellae |
|
|
what kind of adaptions does the true FW crab have for NaCl takeup |
-spatial separation of Cl- absorbing epithelium (thin) and Na+ absorbing epithelium (thick) -Cl- absorbing epithelium is much tighter than Na+ absorbing epithelium -cells might be coupled electrically |
|
|
NaCl excretion model of hypregulating crabs |
basolateral: Na/K/Cl, potassium channel, NKA apical: H/ATPase, chloride channel |
|
|
what is the marine elasmobranch osmoregulatory strategy |
SW osmolality= 1000 mOsm/kg , 400mM Na+ and 450 mM Cl-
plasma osmolality= 940-960 mOsm/kg, Urea 350 mM, Na 286 mM, Cl 296 mM, TMAO 60mM iso-hyporegulators |
|
|
where is urea produced |
in the liver by the ornithine cycle |
|
|
is urea toxic? |
in high concentrations it's toxic to mammals- elasmobranchs retain it as part of their "osmotic ballast" |
|
|
are elasmobranch ureotelic? |
yes |
|
|
what counteracts the toxicity of urea? |
methylamines |
|
|
optimal concentration of urea |
2:1 |
|
|
how does the shark breath |
it enters the gill chambers through the mouth or spiracles in order for the shark to breathe. Blood in the gill filaments absorb oxygen from the water and water then exits through the gill slits. |
|
|
gill modifications in sharks |
low urea permeability -> baso membrane vesicles high cholesterol -baso membrane more permeable to urea than apical -active back transport system |
|
|
what transporter is in the shark gill for urea control |
urea-sodium exchanger |
|
|
osmolality of the shark urine |
800 mOsm/kg, Na 240mM, Cl- 240 mM |
|
|
where is some urea exiting |
through the gills (small bit) |
|
|
adaption of the elasmobranch nephron |
corrosion cast that is very complex and has 4 different loops that transcend 2 distinct regions |
|
|
elasmobranch renal adaptions |
complex tubule - 90% filtered urea reabsorbed -4-20% of urea loss in elasmobranchs -active and passive -facilitative transport of urea |
|
|
what does the rectal gland of sharks produce |
high NaCl fluid |
|
|
osmolality of the rectal gland fluid (fluid will be excreted with the urine) |
osmolality= 940 mOsm/kg, Na 460 mM, Cl- 460 mM |
|
|
NaCl at the gills of sharks |
passive influx vs. active excretion |
|
|
example of a euryhaline elasmobranch |
Atlantic stingray, Bull shark |
|
|
osmoregulatory strategy for FW elasmobranchs |
water= less than 10 mOsm/kg plasma osmolality= 640-670 mOsm/kg, Na225 mM, Cl- 220 mM Urea 193 mM TMAO<30mM |
|
|
osmoregulatory strategy of SW elasmobranchs |
(same as marine before) SW osmolality= 1000 mOsm/kg , 400mM Na+ and 450 mM Cl-plasma osmolality= 940-960 mOsm/kg, Urea 350 mM, Na 286 mM, Cl 296 mM, TMAO 60mM |
|
|
osmoregulation method for frogs |
-belly patch -osmoregulate like FW teleosts |
|
|
what does the tadpole osmoregulate like |
the marine teleost |
|
|
the adult frog retains urea like what |
like a marine elasmobranch fish |
|
|
water loss occurs through what 4 principles |
respiration, cutaneous (integument) evaporation, feces, urine |
|
|
many dessert animals rely on metabolic water produced by what? |
mitochondrial respiration |
|
|
land animal renal adaptions |
-long juxtamedullary nephron -concentrate urine |
|
|
adaptions from desert rats and mice |
-produce feces that are devoid of water -rectal water handling -nasal counter-current mechanism -behaviour aspects- nocturnal ability, burrowing in the day |
|
|
how can fluctuations in body temp be a method of water retention |
reduction of evaporative cooling |
|
|
other methods that camels can retain water |
-eating plants at the right time of day- dew on leaves in the early morning. -drinking a lot in one go -> haemodilution -plasma sparing |
|
|
adaptions for water retention from desert amphibians |
-burrowing or aestivation -cocoon to protect from evaporative loss -bladders act as resevoirs -water absorbed across bladder -low metabolic rate |
|
|
example of post renal modification of urine |
cloacal h2o reabsorption. possible solute linked to reabsorption excess salts then secreted by salt gland |
|
|
what do salt glands allow the animal to do and where are they. |
drink water. nasal, tongue, eyes. -uses Na/K/ATPase |
|
|
describe intestinal reabsorption |
-lack a bladder -uretral urine is refluxed from the cloaca into the colon -secondary water reabsorption -paste-like feces- uric acid |
|
|
How to investigate osmoregulation? |
1. Where is the animal living? SW, intertidal, brackish, FW -consequences of inverts and verts 2. determine osmotic gradients from blood to environment. Need for osmoregulation? epithelium tight for ions? (How would u test this?) 3. potential tissues that might be involved in regulation. ventilated, thin, exposed, MRC, NKA activity changes and mRNA expression, ion transporters at different salinities 4. measurment of ion fluxes (ISME, PDte, Isc, tracer fluxes) 5. which transporters involved? using inhibitors, mRNA, protein |
|
|
how much nitrogen does air have |
78% N2 |
|
|
what animals can use nitrogen in n2 form |
only prokaryotes |
|
|
what secures nitrogen in a fixed form |
plants |
|
|
what do plants store nitrogen as |
compounds such as nitrate (NO3-) , ammonia/ammonium and urea |
|
|
how do animals secure their nitrogen |
from plants that they feed on |
|
|
4 processes of the nitrogen cycle |
nitrogen fixation (N2 to NH3) nitrification (NH3 to NO3-) decay (amino, nucleic acids to NH3) denitrification (NO3- to N2) |
|
|
types of nitrogen fixation |
atmospheric, biological and industrial fixation |
|
|
atmospheric fixation |
energy breaks n2 to form NO and NO2- dissolve in rain to form NO3- |
|
|
biological fixation |
ability only found in some bacteria and archea -symbiotic relationship with plants of legume family -some symbionts with animals (termites) -some in soil |
|
|
what is needed for biological fixation |
Nitrogenase system (enzyme system) -sensitive to O2 |
|
|
what does the nitrogenase system do? |
uses 16 mol ATP/1 mol N2 -> N2, 8H+ , 8e- then nitrogenase reduces ->2NH3, H2 |
|
|
what is used to protect the nitrogenase system against O2 |
hydrogenase- O2 protective- most n2 fixing bacteria Leghemoglobin -o2 binding protein, buffer- in rhizobium species of legumes |
|
|
industrial fixation |
-under high pressure and temperature and the use of a catalyst |
|
|
do plants only use ammonia, ammonium? |
NH4 can be taken up directly by plants by AMT's in their roots but they usually convert it to NO3- by nitrifying bacteria |
|
|
how is nitrification accomplished |
1. Nitrosomonas bacteria oxidize NH4+ to get NO2- 2. Nitrobacter bacteria oxidize the nitrites to nitrates. |
|
|
decay |
metabolism produces organic nitrogen compounds that return to the environment by excretions or death - final beneficiaries are organisms of decay= bacteria and fungi |
|
|
what do organisms of decay do (bacteria and fungi) |
break down nitrogenous molecules in excretions and dead organisms into ammonia |
|
|
nitrogen in excreted in what ways |
ammonia, urea, uric acid |
|
|
denitrification is accomplished by what? |
reduces nitrates to gas- accomplished by anaerobic bacteria living in the soil - they use nitrates as an alternative to oxygen as their final electron acceptor in respiration |
|
|
denitrification cycle |
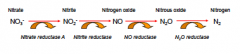
|
|
|
amino acids are used for what? |
energy metabolism, amino acid metabolism, protein anabolism |
|
|
how many amino acids required for protein synthesis |
20-22. Some of them 9-10 are essential and cannot be synthetized, some can be formed from other amino acids |
|
|
General formula of amino acid transfer |
amino acid [amino acid transferase] -> a-keto-acid a-ketogluterate->glutamic acid NAD2+ + H2O [glutamate dehydrogenase] -> NADH + NH4+ *** this is a reversible reaction |
|
|
what can be directly deaminated |
serine [serine-dehydratase] -> pyruvate + NH4+ threonine [threonine dehydratase] -> a-ketobutyrate + NH4+ |
|
|
amino acids feed into the krebs cycle for what |
to produce a lot of energy and ammonia |
|
|
purine catabolism process |
(adenine or guanine) purines [xanthinoxidase] - uric acid [uricase] - allantoin [allantoinase] -allantoic acid - [allantoicase]- 2 urea [urease] -> 4NH4+ and 2 CO2 |
|
|
what has nitrogen end products |
proteins: NH3 -urea and uric acid Nucleic acids: purines and pyrimidines-> NH3
|
|
|
the cellular end product of nitrogen metabolism is... |
ammonia |
|
|
pka of ammonia |
it is a weak base - 9.3 |
|
|
what does the henderson hasslebalch equation say in terms of ammonia |
50% of ammonia occurs exists as NH3 at pH 9.3 |
|
|
when is 99% of ammonia in NH4+ |
at physiological pH 7.3-7.8 |
|
|
what is ammonia responsible for in the body |
amino acid metabolism, energy metabolism, changes in intracellular pH, acts like potassium, neurotoxin, permeably to membranes in gas state. |
|
|
ammonia trapping |
nh4 cant diffuse nh3 diffuses through membrane low intracellular pH, lots of protons nh3 converted to nh4 inside cell |
|
|
how is ammonia toxic? |
all proteins, enzymes etc. have optimal pH. -ionic and hydrogen bonds can be compromised -changes in structure and interactions ammonia can diffuse in acidic vessicles and impair function ammonia can disrupt mitochondrial ATP synthesis by reducing H+ gradient over inner membrane can compete with potassium in any transporters |
|
|
enzyme examples that need optimal pH
|
pepsin : low pH cholinesterase and trypsin: high pH |
|
|
in humans, increased blood ammonia concentration can cause what? |
hepatic encephalopathy |
|
|
normal ciruclating ammonia plasma levels |
< 40uM |
|
|
increased ammonia levels are usually do to what? |
liver failure: urea cycle errors, alcoholism, damage |
|
|
what can hyper ammonia affects cause |
sleep disorder, muscular incoordination, tremors, coma, death |
|
|
LD50 value? |
< 1mM |
|
|
2 hypothesis of mammalian hepatic encephalopathy |
1) excess glutamate neurotransmission, NMDA receptor/ Ca+ hypothesis 2) astrocyte swelling- glutamine accumulation hypothesis |
|
|
post synaptic affects by glutamate neurotransmitter |
1.Glutamate release by activatedpre- synaptic nerve terminalopens non-NMDA glutamatereceptor channels (AMPA)
2. Na+ influx depolarixes post synaptic membrane 3. depolarization removes Mg2+ block from NMDA receptor 4. When glutamate is bound to NMDA receptor, channel opens and allows in Ca+ entry. |
|
|
excess glutamate neurotransmission/ NMDA/ Receptor/ Ca+ hypothesis |
-Nh3 activates NMDA receptor directly? -nh3 inhibits na+ dependent glutamate reuptake into astrocytes? -increased NMDA receptor activation by glutamte leads to increased and uncontrolled Ca+ influx and finally to cell death |
|
|
what can you use to investigate ammonia toxicity via NMDA receptor |
-protective affect of MK-801 against acute ammonia toxicity (use knock outs) -seeing difference in survival rates might bring evidence that NMDA receptor is a part of the toxic mechanism |
|
|
astrocyte swelling- glutamine accumulation hypothesis |
Increased glutamine synthesis by Glutaminesynthetase and glutamineaccumulation in astrocytes
-osmotic cell swelling and brain edema - cell death |
|
|
what does ammonia also affect |
endo-exo cytotosis processes carbohydrate and fat metabolism blood brain barrier |
|
|
can elevated ammonia also be toxic to plants? |
yes |
|
|
what can excess ammonia affect in plants? |
growth, ion balance, acidification of rhizosphere, hormonal balance |
|
|
how can ammonia pass a membrane |
ammonia trapping (diffusion), rhesus, AMT, aquaporins (aquaammoniaporins), vesicular ammonia transport |
|
|
how can ammonium cross the cell membrane |
paracellular pathway from gradient, NKA, cHKA, passive potassium channels by gradient, secondary active NA/K/Cl and NHE, H+ATPase protons react with diffuesed nh3, rhesus, AMT, vessicular transport |
|
|
how does NHE work for ammonium transport |
pumps protons and reacts with nh3 that diffused through membrane |
|
|
three isoforms of rhesus transporters |
Rhag: red blood cells Rhbg: basolateral Rhcg: apical -they all form trimers |
|
|
how does Rhcg work |
electroneutral nh3 transport |
|
|
how does Rhag and Rhbg work |
co-transport of nh3 and h+ (in trimers, h+ can fit through) |
|
|
AMT ammonia transporter |
- ex) in plant roots for ammonia and ammonium transport transport not known: -three ammonia transporters (trimer) -ammonia fitting through in pore between them -or electrogenic nh4+ transport through transporters |
|
|
are AMT's in vertebrates? |
no, they are in inverts, bacteria, plants |
|
|
exception to ammonotelism aquatic trend |
elasmobranchs excrete mainly urea |
|
|
exception to land living animals not excreting ammonia |
some crabs, blood feeding insects, mosquito larva, |
|
|
animals with excretion transitions |
dragon fly: ammonia as larva, uric acid as adult some amphibians: ammonia as tadpole, urea as adult |
|
|
what do aquatic amphibians excrete as adults |
ammonia. |
|
|
ammonia excretion in marine organisms |
some diffusion of nh3 basolateral: NKA, Na/K/Cl, Rhbg apical: Rhcg, NHE, H+ATPase |
|
|
ammonia excretion in FW |
-depends on gill boundary acidification basolateral: NKA, Rhbg apical: NHE -helping to acidify gill boundary, Rhcg, H+ATPase[major player] |
|
|
does ammonia excretion decrease or increase with increasing pH |
decreases |
|
|
how can you localize the transporters |
immunohistochemistry, in-situ hybridization |
|
|
what happens to fish in alkaline waters |
increased alkalinity reduced the number of available H+ and therefore reduces NH3 gradient across the gills |
|
|
how do we investigate ammonia excretion? |
how feasible is it to investigate ammonia transporters in animal of interest? -What techniques can I apply? -Can I take blood samples (does size allow)? -What is know about the gas exchange and ion transport epithelia? |
|
|
example of investigation C. maenas. what do we know about c. maenas? |
• Ammonotelic?
• Carnivorous -> High protein metabolism? • Habitat (benthic, marine and brackish water) • Main site of ammonia excretion is the gill epithelium • Morphology of transport-epithelium? • How feasible is it to investigate branchial ammoniatransports in C. maenas ? |
|
|
what else do we have to know before running experiments? |
ammonia concentration of the blood to operate in the physiological range |
|
|
what kind of experiment would you do to identify a transporter-mediated excretion mechanism? |
-check if excretion is active -measure flux |
|
|
what kind of experiment would help identify active transport mechanisms |
symmetric conditions- no driving gradients ammonia increase on outside and decrease on hemolymph side |
|
|
how to measure metabolic ammonia? |
0:0 |
|
|
investigating ammonia mechanisms? |
using inhibitors, localizations, western blotting for transporters in tissue, complementation assay |
|
|
describe the complementation assay |
yeast growth on media containing ammonia as the single nitrogen source. -check if yeast grows with addition or removal of transporters (genetic modification) |
|
|
how can ureotelism be induced |
feeding, air exposure, stress, environmental ammonia |
|
|
amphibian renal handling of urea |
-most urea filtered in nephron - tubule poorly permeable to urea, so most ends up in urine -concentrated from water being reabsorbed - |
|
|
how much urea in the plasma of mammals |
4-10 mM |
|
|
where is urea produced |
liver |
|
|
where is urea filtered |
kidney |
|
|
pH is regulated by what buffers |
carbonate, weak acid/bases, proteins, NH4+ |
|
|
physiological pH |
7.3-7.8 |
|
|
responses for regulating pH |
buffering, respiratory compensation, ionic shuffling between fluids and environment |
|
|
internal fluid homeostasis is largeley regulated by what |
carbonate buffering equilibrium |
|
|
determining factor of intracellular pH |
proteins |
|
|
acid base distrubances |
respiratory alkalosis and acidosis, ketoacidosis, lactic acidosis, renal tubular acidosis |
|
|
what reabsorbes bicarbonate from the blood |
the kidney. the kidney generate bicarbonate and excretes ammonia |
|
|
explain the proximal tubule in pH regulation |
-protons pass into tubular fluid by apical NHE and H+/ATPase bicarb is moved to the blood by NBCC and bicarb/cl- transporters -carbonic anhydrase is crucial here, transforming H2CO3 into co2 and water |
|
|
what grabs free bicarbonate |
volatile acids |
|
|
ammoniagenesis: nh4 and bicarbonate |
-glutamate metabolized to form 2NH4+ and 2bicarbonate -ammonium goes into urine - bicarbonate goes to the blood -left over ammonium is converted by the liver to urea -makes more free protons and annulates the bicarb production |
|
|
fishy acid base regulation- osorezandace |
pH- 3.5, freshwater NKA pumps out Na, neg cell membrane potential environmental na pulled to the neg potential using NHE reduced pH. from proton loss increases bicarb, carbonic anhydrase bicarb and negative cell potential drives NBC (sodium bicarb cotransport) to buffer blood |
|
|
transporters involved in co2 excretion |
NKA, potassium/ammonium channels, Rh, bicarb/sodium exchanger, carbonic anhydrase |

