![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
What are the units of Enthalpy? |
Kilojoules per mole kJmol⁻¹ |
|
|
What is meant by the word "system"? |
-The reactants and products -can lose or gain energy |
|
|
What is meant by the word "surroundings"? |
The rest of the world, i.e. the test tube, air |
|
|
Define ∆Hcº |
The enthalpy change that occurs when one mole of a substance completely burns in excess oxygen under standard conditions. |
|
|
State Hess' Law |
The enthalpy change for ay substance is independent of the intermediate stages, so long as the initial and final conditions are the same for each route. |
|
|
Define endothermic reaction, including energy change diagram |
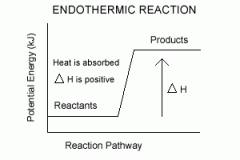
Enthalpy of reacting system increases, takes in energy from surroundings. |
|
|
Define ∆Hrº |
The enthalpy change when molar quantities of reactants as stated in the equation react together under standard conditions. All the substances are in their standard states. |
|
|
What are the standard conditions? (with units) |
-Specified temperature --> 298ºK -Standard pressure of 1 atomsphere --> 1.01x10⁵ N m⁻² -Standard concentration of 1 mold dm⁻³ |
|
|
Define exothermic reaction, including energy change digram. |
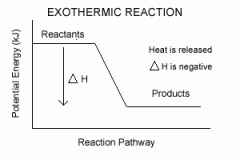
Enthalpy of reacting system decreases, gives out het and energy to the surroundings. |
|
|
Define ∆Hfº |
The standard enthalpy change one mole of a compound is formed from its constituent elements, with both the compound and elements in their standard states. |
|
|
Symbol for enthalpy and its definition. |
∆H The measure of energy transferred to and from the surroundings. |
|
|
What equation do we use to work out the energy transferred? |
-∆H = products - Hreactants -q= mc∆T -q= energy transferred -m= mass of substance being heated by the fuel -c= speciific heat capacity (4.18 for water) -∆T= change in temperature of substance being heated. |

