![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
140 Cards in this Set
- Front
- Back
|
Normal parathyroid histology:
|
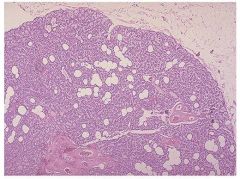
Normal parathyroid gland with admixed fat.
|
|
|
What type of cells are parathyroid glands mostly composed of?
|
Chief cells; these cells vary from light to dark pink and contain secretory granules of PTH.
|
|
|
How is the secretion of PTH from the parathyroid glands controlled?
|
The activity of the parathyroids is controlled by the level of free calcium in the blood rather than by trophic hormones secreted by the pituitary or hypothalamus.
|
|
|
Decreased levels of free calcium in the blood would increase or decrease PTH secretion?
|
Increase
|
|
|
What are the actions of parathyroid hormone?
|
1. Activate osteoclasts, thereby mobilizing calcium from bone.
2. Increase the renal tubular reabsorption of calcium. 3. Increase the conversion of vitamin D to its active form in the kidneys. 4. Increases urinary phosphate excretion. 5. Augment GI calcium absorption. |
|
|
What is the difference between primary and secondary/tertiary hyperparathyroidism?
|
Primary hyperparathyroidism results from spontaneous overproduction of PTH.
Secondary and tertiary hyperparathyroidism are a result of chronic renal insufficiency. |
|
|
What are the three underlying causes of hyperparathyroidism and how common are each of them?
|
1. Adenoma (75-80%)
2. Primary hyperplasia (10-15%) 3. Parathyroid carcinoma (less than 5%) |
|
|
What is familial hypocalciuric hypercalcemia?
|
A rare disorder caused by mutations in the calcium sensing receptor gene (CASR) on parathyroid cells leading to constant PTH secretion.
|
|
|
What two mutations are common in parathyroid tumors?
|
PRAD1 and MEN1
|
|
|
Parathyroid adenoma histology:
|
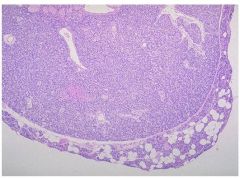
Parathyroid adenomas are composed mostly of chief cells. A rim of compressed, non-neoplastic parathyroid tissue, generally separated by a fibrous capsule, is often visible at the edge of the adenoma. In contrast to the normal parathyroid parenchyma, adipose tissue is inconspicuous within the adenoma.
|
|
|
Are parathyroid adenomas usually multi-glandular or confined to a single gland?
|
Confined to a single gland.
|
|
|
Is parathyroid hyperplasia usually multi-glandular or confined to a single gland?
|
Usually a multiglandular process.
|
|
|
Parathyroid hyperplasia histology:
|
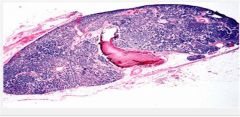
Chief cell hyperplasia with a decrease in stromal fat.
|
|
|
Parathyroid carcinoma histology:
|
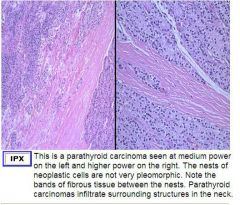
Usually firm or hard tumors occuring in one gland. Usually larger than adenomas.
|
|
|
What are some of the clinical features of primary hyperparathyroidism?
|
1. Increased serum ionized calcium.
2. Inappropriately increased or decreased PTH (depending on cause) 3. Hypophosphatemia 4. Bone fractures 5. Renal stones 6. GI disturbances 7. CNS alterations and neuromuscular abnormalities 8. Polyuria and polydipsia |
|
|
How does chronic renal failure cause secondary hyperparathyroidism?
|
Chronic renal failure leads to decreased phosphate excretion --> hyperphosphatemia.
Elevated serum phosphate depresses serum calcium levels and lead to increased PTH secretion. Renal failure also reduces the kidney's ability to activate vitamin D --> decreased GI absorption of calcium. |
|
|
What are some clinical features of secondary hyperparathyroidism?
|
1. Bone abnormalities are less severe than that in primary hyperparathyroidism.
2. Serum calcium remains near normal b/c a compensatory increase in PTH sustain serum calcium. 3. Parathyroid glands are hyperplastic. |
|
|
Hypoparathyroidism causes:
|
Much less common than hyperparathyroidism.
1. Surgical ablation 2. Congenital absence (usually in conjunction with thymic aplasia and cardiac defects in DiGeorge syndrome) 3. Autoimmune hypoparathyroidism (a hereditary polyglandular deficiency syndrome) |
|
|
Clinical features of hypoparathyroidism?
|
1. Increased neuromuscular irritability.
2. Cardiac arrhythmias 3. Increased intracranial pressure and seizures. 4. Cataracts and dental abnormalities. |
|
|
BMI calculation formulas:
|
Either weight in Kg / height in meters squared OR
weight in lbs * 703 / height in inches squared |
|
|
A BMI of _____ or over is considered obese?
|
30
|
|
|
A BMI of _____ to ______ is considered overweight?
|
25 to 30
|
|
|
What does the OB gene code for?
|
Leptin; a protein that decreases food intake and increases energy expenditure.
|
|
|
Mutations in POMC can cause obesity via failure to produce waht neuropeptide that inhibits appetite?
|
alpha-MSH
|
|
|
This enzyme in fat cells can convert inactive cortisone to cortisol:
|
11B-hydroxysteroid dehydrogenase
|
|
|
What are the four pancreatic hormones and what cells do they come from?
|
1. B cells: insulin
2. A cells: glucagon 3. D cells: somatostatin; suppresses both insulin and glucagon release 4. PP cells: vasoactive intestinal peptide; stimulates secretion of gastric and intestinal enzymes and inhibition of intestinal motility. |
|
|
Normal blood glucose level is?
|
Between 70 - 120 mg/dL
|
|
|
The diganosis of diabetes is established by elevation of blood glucose by any one of 3 criteria:
|
1. A random blood glucose level of 200 or higher, with classical signs and symptoms
2. A fasting glucose concentration of 126 or higher on more than one occasion. 3. An abnormal oral glucose tolerance test in which the glucose level is 200 or above 2 hours after a standard dose of 75mg of glucose. |
|
|
What serum fasting glucose levels or oral glucose tolerance test levels would be considered "impaired glucose tolerance"?
|
111-125 for fasting serum levels.
141-199 for OGTT levels. |
|
|
Type I diabetes:
|
Characterized by an absolute deficiency of insulin secretion caused by pancreatic B-cell destruction, usually resulting from an autoimmune attack. Type 1 diabetes accounts for about 10% of all cases.
|
|
|
Type II diabetes:
|
Caused by a combination of peripheral resistance to insulin action and an inadequate compensatory response of insulin secretion by the pancreatic B-cells. About 80-90% of all cases of diabetes are type II
|
|
|
Long-term organ complications in diabetes include?
|
1. Kidneys
2. Eyes 3. Nerves 4. Blood vessels |
|
|
The primary metabolic function of insulin is what?
|
To increase the rate of glucose transport into certain cells in the body; the striated muscle cells (including myocardial cells) and to a lesser extent, adipocytes.
|
|
|
Several mechanisms may contribute to B-cell destruction in type I diabetes:
|
1. Th1 and cytotoxic T cell damage due to reaction against B-cell antigens
2. Locally produced cytokines damage B-cells 3. Autoantibodies to B-cell antigens |
|
|
A person has a greatly increased susceptibility to diabetes type I with these Type II MCH genes:
|
HLA-DR3 and HLA-DR4
HLA- DR2 actually has a protective effect. |
|
|
Genetic factors play more of a role in which type of diabetes?
|
Type II
|
|
|
The two metabolic defects that characterize type 2 diabetes are?
|
1. A decreased ability of peripheral tissues to respond to insulin
2. B-cell dysfunction that is manifested as inadequate insulin secretion in the face of insulin resistance and hyperglycemia. |
|
|
There is what type of relationship between free fatty acids and insulin in the blood?
|
An inverse relationship
|
|
|
What effects does thiazolidinedione have on PPARg in adipose cells?
|
TZD targets the PPAR receptor in adipose cells: PPAR activation by TZD results in changes in production of adipocytokines and a lowering of FFA's in the blood.
This results in a decrease in insulin resistance. |
|
|
What is monogenic diabetes?
|
A diabetic pheontype occuring secondary to loss-of-function mutations within a single gene.
|
|
|
Name the three major metabolic pathways that contribute to the complications of diabetes?
|
1. Non-enzymatic glycosylation
2. Activation of protein C |
|
|
Name the three major metabolic pathways that contribute to the complications of diabetes?
|
1. Non-enzymatic glycosylation
2. Activation of protein C |
|
|
Name the 3 major metabolic pathways that contribute to the complications of diabetes:
|
1. Non-enzymatic glycosylation
2. Activation of protein C 3. Intracellular hyperglycemia with disturbances in polyol pathways. |
|
|
How does non-enzymatic glycosylation cause problems in diabetes?
|
Through the formation of "advanced glycosylation end products"; these accumulate in the interstitial tissue and blood vessel walls. This process may trap LDL in vessel walls, enhancing cholesterol deposition in the intima, accelerating atherosclerosis.
Albumin will bind to glycosylated kidney proteins --> thickening of the basement membrane. AGE receptors on macrophages and other cells contribute to increased inflammatory and procoagulant activity. |
|
|
How does activation of protein kinase C occur in diabetes?
|
Intracellular hyperglycemia induces de novo synthesis of DAG --> activation of PKC.
PKC activation may cause neovascularization of the retina (diabetc retinopathy) and increased deposition of extracellular matrix and basement membrane material. |
|
|
How does a disturbance in the polyol pathway in cause damage to nerves, kidneys, lens and blood vessels in diabetes?
|
Increased use of the polyol producing aldose reductase pathway in these tissues reduces antioxidant reserves leaving them vulnerable to damage.
|
|
|
Characterization of Type I diabetes from Robbins:
|
An autoimmune disease characterized by progressive destruction of islet B-cells, leading to an absolute insulin deficiency. Several immune mechanisms probably contribute to B-cell damage, including cytokines, T cells, and autoantibodies.
|
|
|
Characterization of Type II diabetes from Robbins:
|
Has no autoimmune basis. Instead, features central to its pathogenesis are insulin resistance and B-cell dysfunction, resulting in relative insulin deficiency.
|
|
|
Obesity relationship with Type II DM from Robbins:
|
Obesity has an important relationship with insulin resistance, probably mediated by cytokines released from adipose tissues (adipocytokines). Other players in the adipo-insulin axis include free fatty acids and the PPARg receptor which modulates adipocytokine levels.
|
|
|
Structures most likely to be damaged in even well controlled type I or II DM:
|
1. Arteries
2. Basement membranes of small vessels 3. Kidneys 4. Retinas 5. Nerves |
|
|
Pancreatic cellular changes in type I DM:
|
Reduction in the number and size of islets.
Leukocytic infiltration of the islets. |
|
|
Pancreatic cellular changes in type Ii DM:
|
A subtle reduction in islet cell mass.
Amyloid replacement of islets in long standing disease; fibrosis may also occur. |
|
|
Diabetic macrovascular disease:
|
A hallmark is accelerated atherosclerosis affecting the aorta and large and medium sized arteries.
Greater severity and earlier onset in diabetics. Contributes to most common cause of death in diabetics; MI. Contributes to gangrene of the lower extremities; also more common in diabetics. |
|
|
Diabetic microangiopathy:
|
Diffuse thickening of basement membranes in capillaries of skin, skeletal muscle, retina, renal glomeruli and renal medulla as well as renal tubules, Bowman capsule and peripheral nerves.
Thickening is due to collagen type IV deposition in the basal lamina. Despite the increase in the thickness of basement membranes, diabetic capillaries are more leaky than normal to plasma proteins. |
|
|
Which disease is the second most common cause of death in diabetics?
|
Renal failure
|
|
|
Diabetic nephropathy:
|
Three types of damage are found in this disease:
1. Glomerular lesions 2. Renal vascular lesions 3. Pyelonephritis |
|
|
Types of glomerular lesions found in diabetes:
|
1. Capillary basement membrane thickening.
2. Diffuse mesangial sclerosis. - A diffuse increase in mesangial matrix and mesangial cell proliferation. - When disease is advanced; patients manifest nephrotic syndrome - proteinuria, hypoalbuminemia and edema. 3. Nodular glomerulosclerosis. - A ball-like lesion situated in the periphery of the glomerulus. Always associated with diabetes. Can induce sufficient ischemia to cause scarring of the kidneys. |
|
|
What type of acute pyelonephritis is more common in diabetics?
|
Necrotizing papillitis (papillary necrosis)
|
|
|
Ocular complications of diabetes:
|
May take the form of retinopathy, cataract formation or glaucoma.
Nonproliferative retinopathy: - characterized by capillary thickening and hemorrhage; may see small red dots. Proliferative retinopathy: - Will see fibrosis and neovascularization with possible retinal detachment. |
|
|
Diabetic neuropathy:
|
Characterized by a peripheral, symmetric neuropathy of both motor and sensory function in the lower extremities.
May also cause disturbances in bowel, bladder, and sexual function. Damage is caused by microangiopathy of the vessels supplying the nerves and by direct axonal damage due to alterations in polyol metabolism. |
|
|
What makes up the "classic triad" of diabetes?
|
Polyuria, polydipsia and polyphagia.
|
|
|
How and why are ketone bodies produced?
|
Insulin deficiency leads to activation of hormone sensitive lipase, with resultant excessive breakdown of adipose stores, giving rise to increased levels of FFA's, oxidation of which by the liver produces ketone bodies.
|
|
|
Type I and II DM comparison table:
|

|
|
|
How does production of ketone bodies lead to ketoacidosis?
|
The rate at which ketone bodies are formed may exceed the rate at which they can be used, leading to ketonuria and ketonemia. If the urinary excretion of ketones is compromised by dehydration, the plasma hydrogen ion concentration increases, resulting in metabolic ketoacidosis.
|
|
|
What is hyperosmolar nonketotic coma and in what population does this develop?
|
Elderly type II diabetics who cannot drink enough water to compensate for the water loss from osmotic diuresis may develop this - basically severe dehydration leading to coma.
|
|
|
What are the 3 top causes of mortality in both Type I and II diabetics?
|
1. MI
2. Renal vascular insufficiency 3. Cerebrovascular accidents |
|
|
What is the earliest manifestation of diabetic nephropathy?
|
Microalbuminuria; small amounts of albumin in the urine.
|
|
|
Diagram of how ketoacidosis develops:
|
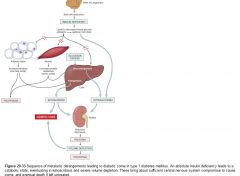
|
|
|
What are the 6 important organs in calcium homeostasis?
|
Bone, Liver, Kidney, GI, Parathyroid and Skin
(BLK-GPS) |
|
|
Levels of calcitonin are useful for detecting what?
|
Medullary carcinoma of the thyroid.
|
|
|
Vitamin D levels:
|

|
|
|
Vitamin D effects:
|
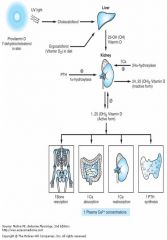
|
|
|
What type of bone is the axial skeleton made of? The peripheral bones?
|
Axial skeleton is made mostly of trabecular bone. The peripheral bones are made mostly of cortical bone.
|
|
|
What effect does PTH have on phosphate levels?
|
1. PTH increases bone resorption leading to an increase in serum calcium AND phosphate.
2. PTH inhibits renal phosphate reabsorption in the proximal tubule and increases calcium reabsorption in the distal tubule - leading to decreased serum phosphate levels. |
|
|
Cycle of hypercalcemia and dehydration:
|
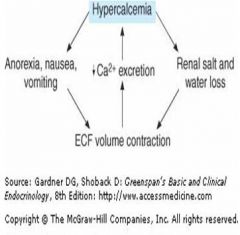
|
|
|
What is the mnemonic for hyperparathyroid symptoms?
|
Stones, bones, abdominal groans and psychic moans.
|
|
|
Signs and symptoms of hyperparathyroidism:
|
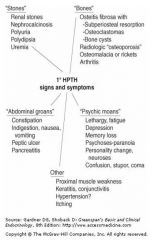
|
|
|
Under the influence of PTH, where is most bone resorbed?
|
From the surface of cortical bones.
|
|
|
Which type of bone benefits most from calcium supplementation in a patient with osteoporosis?
|
Trabecular bone
|
|
|
Guidelines for surgical intervention in hyperparathyroidism:
|
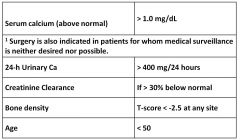
|
|
|
Monitoring guidelines for silent hyperparathyroidism:
|
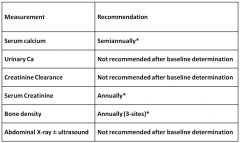
|
|
|
Signs and symptoms of hypoparathyroidism:
|

|
|
|
A carpal spasm induced by compression of the ulnar nerve or occlusion of the circulation is called _________ sign and is indicative of what?
|
Trousseau's sign; indicative of hypocalcemia.
|
|
|
Hyperactivity of the facial nerve when tapped is called _________ sign and is indicative of what?
|
Chvostek's sign; indicative of hypocalcemia.
|
|
|
Short stature and shortened 4th and 5th fingers are a sign of what hereditary disease?
|
Pseudohypoparathyroidism
|
|
|
What is the pathophysiology of psuedohypoparathyroidism?
|
This disease is the result of a defective Gs protein in kidney and bone which causes end organ resistance to PTH.
- Hypocalcemia and hyperphosphatemia occur and are not corrected with administration of PTH. - Endogenous PTH levels are elevated. |
|
|
How much calcium of the total in the body is not in the bone?
How much of that is free calcium? |
1%
1/2 |
|
|
Calcium and phosphate are complexed together in the form of ___________ crystals:
|
Hydroxyapatite
|
|
|
What is the effect of pH on calcium serum levels?
|
Hydrogen competes with calcium for binding sites on albumin.
- In an acidosis, there are more hydrogen ions --> more hydrogen ions attached to albumin and more free calcium --> increased calcemia - In alkalosis, there is less hydrogen ions to compete with calcium, so more calcium is bound to albumin --> decreased free calcium and lowered calcemia. |
|
|
What serum calcium levels would you probably see in an acidosis? An alkalosis?
|
Acidosis = hypercalcemia
Alkalosis = hypocalcemia |
|
|
What are the 3 types of cells contained in the parathyroid gland?
|
1. Chief cells
2. Oxyphil cells 3. Clear cells |
|
|
What else other than low calcium can stimulate the parathyroid gland to produce PTH?
|
Hyperphosphatemia
|
|
|
How are chief cells stimulated to produce PTH?
|
Parathyroid cells contain Ca level sensing receptors.
If extra cellular levels of Ca are high, Ca binds to receptors, activates phospholipase C which inhibits PTH production. If Ca level is low, there is less receptor binding, serves as a stimulant for PTH secretion. |
|
|
Other conditions associated with hypercalcemia:
|
Hyperparathyroidism
Malignancy (Breast, lung) Multiple Myeloma Osteoporosis Sarcoidosis Lab error Prolonged immobilization Prolonged ischemia Thiazide diuretics Vit A overdose |
|
|
What is PTHrP and what is it a marker for?
|
Parathyroid Hormone related Protein; a marker for solid tumors.
Produces the same effect as PTH on bone and kidney and causes disease like primary hyperparathyroidism. |
|
|
Hypercalcemia in multiple myeloma occurs why?
|
Occurs by upregulation of RANKL which activates osteoclasts (osteclast activating factor)
|
|
|
Excess of what vitamin can cause activation of osteoclasts?
|
Vitamin A
|
|
|
Besides the Chvostek and Trousseau signs, what are some other manifestations of hypocalcemia?
|
Confusion, seizures,
Weakness, cramping, spasms Prolonged Q T, CHF Laryngospasm, stridor |
|
|
In humans, calcitonin has little effect on regulating calcium - so what is measuring it good for?
|
As a tumor marker:
(best way to diagnose) Medullary carcinoma of the thyroid, a malignant tumor of C cells (parafollicular cells) |
|
|
What is metabolic syndrome?
|
Having metabolic syndrome means you have three or more disorders related to your metabolism at the same time, including:
Obesity, particularly around your waist (having an "apple shape") SBP>120 mmHg or a diastolic BP> 80 mm Hg An elevated level of triglycerides and a low level of high-density lipoprotein (HDL) cholesterol Resistance to insulin |
|
|
What role in obesity does the melanocortin 4 receptor play?
|
MC4R mutation accounts for 5% of individuals with childhood onset obesity. The actions of melanocyte-stimulating hormone on the melanocortin 4 receptor (MC4R) lead to a decrease in food intake, and mice with null mutations in MC4R have increased food intake, obesity, and hyperinsulinemia.
Binge eating is the common path phenotype for these mutations. |
|
|
MSH comes from a primary POMC transcript and leptin to some degree regulates POMC production - what would the effect of low leptin be?
|
POMC is regulated in part by leptin. Loss-of-function mutations in POMC gene lead to obesity in mice and humans.
Leptin and leptin receptor mutations have been identified in a few families Homozygotes: severe early obesity and hyperphagia Heterozygotes: low leptin and high weight and can be treated with injectable leptin |
|
|
Dopamine helps regulate feeding patterns:
|
Obese have lower levels of the post-synaptic dopamine D2R receptor, associated with an allele called Taq I A. Noted in 20% of obese young women.
Consistent with finding that binge eaters have low dopamine metabolite concentrations in the CSF. Dopamine agonists are anorexigenic in the clinical situation. |
|
|
High levels of dopamine can also induce feeding through stimulation of pleasure centers.:
|
Dopaminergic reward pathways provide the pleasure drive for eating as well as the rewards associated with cocaine, alcohol and nicotine.
|
|
|
What are endocannabinoids and what role do they play in weight regulation?
|
Endogenous lipids that bind to cannabinoid receptors 1 and 2 (CB1, CB2)
CB1 receptors are in hypothalamus, adipose and gi tract tissue Stimulation of CB1 leads to increased oral intake and blockade leads to weight loss CB1 receptors are expressed in the known hedonic centers (nucleus accumbens, hippocampus, and entopeduncular nucleus) |
|
|
What part of the hypothalamus integrates signals concerning satiety and hunger?
|
The arcuate nucleus.
|
|
|
Hunger/Satiety signals and their MOA'a:
|
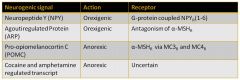
|
|
|
What is leptin?
|
Leptin is a product of the ob gene in adipocytes… an “adipokyne”
It is the molecular signal of energy abundance Serum concentrations of leptin and circulating mRNA are in proportion to body fat mass A decrease in leptin results in a strong hunger signal and obesity, hyperphagia, infertility, and impaired T-cell–mediated immunity in mice and humans Administration of leptin completely reverses all aspects of the phenotype in both species. Plays a role in the Prader-Willi syndrome. |
|
|
How does leptin work?
|
Leptin drives ANOREXIA by:
Blocking NPY/AgRP axis by directly decreasing release of these molecules. Activating POMC/CART axis via gene transcription. |
|
|
Foregut signals for hunger/satiety:
|
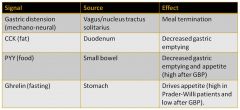
|
|
|
What is Ghrelin?
|
Signals energy insufficiency.
Secreted from endocrine cells of the stomach fundus. Preprandial rise and postprandial decline in circulating levels. Fasting causes persistent elevations. Prader-Willi patients (voracious hyperphagia) have elevated ghrelin levels. |
|
|
Resistin contributes to insulin resistance and DM Type II:
|
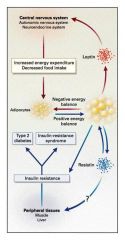
|
|
|
Deaths from what 2 types of cancers are increased in obese men?
3 in women? |
Men: Liver and pancrease
Women: Uterus, kidney and cervix |
|
|
Criteria for diagnosing diabetes according to Dr. Davey:
|
Hemoglobin A1c ≥ 6.5%* using quality lab testing
OR Fasting Plasma Glucose (FPG) ≥ 126 mg/dl. * OR Two hour plasma glucose ≥ 200 mg/dl during an Oral Glucose Tolerance Test (OGTT).* OR In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dl. *In the absence of unequivocal hyperglycemia, criteria 1-3 should be confirmed by repeat testing |
|
|
What are the criteria for testing for diabetes in asymptomatic individuals?
|
Testing should be considered in all adults who are overweight and have additional risk factors:
First-degree relatives with diabetes Physical inactivity Members of high risk ethnic population (Latino, Native American) Women having delivered a baby weighing > 9lb or had GDM Hypertension (≥ 140/90mmHg or on antihypertensive therapy) HDL cholesterol <35 mg/dl or triglycerides >250 mg/dl Women with PCOS A1c ≥ 5.7%, IGT, or IFG on previous testing Other clinical conditions associated with insulin resistance History of CVD |
|
|
If a person has no risk factors for diabetes, when should screening begin?
|
In the absence of the above criteria, testing for diabetes should begin at age 45 years.
If results are negative, repeat testing every 3 years, with consideration of more frequent testing depending on initial results and risk status |
|
|
What test results for fasting glucose, glucose tolerance and A1c would indicate pre-diabetes?
|
FPG 100 mg/dl-125 mg/dl: Impaired fasting glucose [IFG]
2-h PG in the 75 gm OGTT 140 mg/dl-199 mg/dl: Impaired glucose tolerance [IGT] A1C 5.7-6.4% |
|
|
Four types of auto-antibodies found in type I DM:
|
GADA: glutamic acid decarboxylase autoantibodies
ICA: Islet cell cytoplasmic autoattibodies IA-2A: Insulinoma associated-2 autoantibodies IAA: Insulin autoantibodies |
|
|
Pathogenesis of DM Type II
|
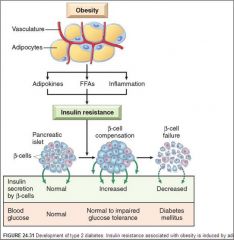
|
|
|
Manifestations of Beta-Cell Dysfunction in Type 2 Diabetes Mellitus:
|
1. Abnormal pulsatile insulin response (In normal secretion, a spike of insulin is secreted followed shortly by a smaller amount of insulin; this is called “first phase”. In hyperglycemia, the first phase secretion is blunted.)
2. Decreased beta-cell responsiveness to glucose 3. Increased proinsulin to insulin ratio 4. Decreased insulin production: - Decreased insulin content - Decreased insulin granule density |
|
|
What is the relationship between atherosclerosis and insulin resistance?
|
Insulin resistance, resulting from genetic and environmental factors, including overnutrition, leads to vasoconstriction, inflammation, and thrombosis.
Inflammation and insulin resistance are reciprocally reinforced, and result in a cycle of accelerated atherosclerosis and increased insulin resistance. |
|
|
What is the only anti-atherogenic adipokine?
|
Adiponectin
|
|
|
Adverse effects of ectopic fat:
|
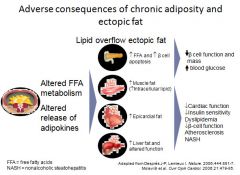
|
|
|
Effects of insulin resistance and visceral obesity:
|
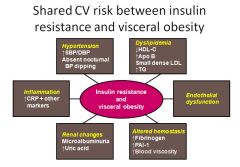
|
|
|
Relationship between type II diabetes and insulin resistance:
|
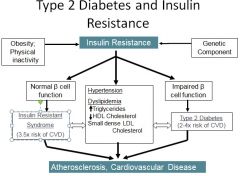
|
|
|
Glucagon stimulates what enzyme to increase hepatic glycogen breakdown?
|
Glycogen phosphorylase
|
|
|
What is the main difference in the cause of ketoacidosis and hyperosmolar hyperglycemic coma?
|
KA is caused by decreased glucose utilization due to insulin deficiency.
HHNS is caused by increased glycogenolysis due to decreased insulin sensitivity. |
|
|
How does a lack of insulin promote the formation of ketone bodies?
|
1. High ratio of glucagon in the portal vein favors the oxidation of fatty acids and formation of ketone bodies.
2. Glucagon blocks glycolysis and decreases availabililty of intermediates required for synthsis of malonyl-coA --> preferential formation of acetyl-coA --> production of ketone bodies |
|
|
Why are endothelial cells effected more by hyperglycemia than other body cells?
|
Because endothelial cells are not able to regulate the amount of glucose they bring in as glucose levels increase, unlike other body cells.
|
|
|
According to Dr. Crockett, what are the 2 mechanisms for developing insulin resistance in obesity?
|
1. Adipose cells release FFA's and leptin --> decrease in post insulin receptor signalling.
2. Adipose cells release TNF-a --> decrease in insulin receptor kinase activity. |
|
|
What is the pathogenesis of dyslipidemia in type II diabetics?
|
HDL breakdown is accelerated leading to high levels of free ApoA protein. ApoA is excreted in the urine faster than it can be absorbed --> net loss.
Pathway now shunts over to mostly LDL production. LDL's are used to make "Small dense" LDL which is highly atherogenic. |
|
|
Diagram of the pathogenesis of vascular disease in diabetes:
|
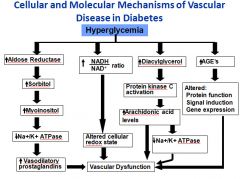
|
|
|
AMA diabetes diagnosis criteria (yet another one):
|

|
|
|
Diabetic ketoacidosis mortality rate?
|
10%
|
|
|
What are some environment triggers for DM?
|

|
|
|
Progression of autoantibody appearance in DM type I
|
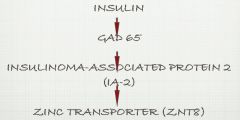
|
|
|
Is a single autoantibody sufficient to cause DM?
|
No, development of multiple autoantibodies is necessary for development and progression of disease.
|
|
|
What type of hyperpigmentation might you see on a Type II diabetic?
|
Acanthosis nigricans:
is often associated with conditions that increase your insulin level, such as type 2 diabetes or being overweight. If your insulin level is too high, the extra insulin may trigger activity in your skin cells. This may cause the characteristic skin changes. |
|
|
Anion gap calculation:
|
AG = [Na+] - [Cl-+ HCO3-]
|
|
|
Corrected serum sodium calculation:
|
Corrected [Na+] =
([Na+] + 1.6 x glucose (mg/dL) – 100) / 100 |

