![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
143 Cards in this Set
- Front
- Back
|
Compare the synthesis of peptide/polypeptide hormones, lipid derivative hormones and amine hormones. Give an example of each.
|
Peptide/polypeptide hormone synthesis: mRNA preprohormone --> signal sequence cleaved off in ER to prohormone --> move through golgi --> cleaved to active hormone in golgi --> packed into secretory vesicles and STORED for later release; INSULIN
Lipid Derivative hormone synthesis: cholesterol is in the cell (from HSL action on lipid droplet) and is taken into the mitochondria by STAR protein --> cholesterol converted to pregnenolone by CYP11A --> --> --> used immediately NOT STORED; STEROIDS **note- ACTH upregulates the action of HSL to break down lipid drops to free cholesterol and upregulates STAR protein Amine hormone synthesis: synthesized from precursor and STORED; T3/T4, catecholamines |
|
|
Compare the half lives of peptide/polypeptide hormones, steroids and amine hormones. Why is this?
|
peptide/polypeptide- water soluble, not protein bound --> short half life
steroids and amines are not water soluble, so they are bound to proteins --> longer half life |
|
|
Where are the cell signaling mechanisms located for peptide hormones? Steroid hormones?
|
Peptide hormones use cell surface receptors.
Steroid hormones use intracellular (nuclear) receptors. |
|
|
List some examples of hormones that use:
GPCR Tyrosine Kinase Nuclear Receptors |
GPCR: FLAT ChAMP (from FA), her specific examples were FSH, LH, TSH, MSH, PTH, glucagon, TRH, catecholamines
Tyrosine Kinase- insulin, GH, prolactin Nuclear Receptors (aka steroid receptors)- vit D, T3/T4 |
|
|
How can desensitization occur with cell signaling? (4)
|
1. Uncoupling GPCR from G protein after binding
2. Endocytosis 3. Modification 4. Decrease Expression |
|
|
What are the lobes of the pituitary and how do they communicate with the hypothalamus?
|
Anterior pituitaryadenohyphysis: direct connection via portal system- minute amounts of hormone are produced by hypothalamus have large effects due to direct connection via vascular plexus
Posterior pituitary/neurohyphysis: neural connection- nerves bodies from SON and PVH produce ADH and oxytocin, respectfully, and the hormones travel down axons and terminate in the posterior pituitary |
|
|
How is ADH secreted, what prompts its secretion and what are its actions?
|
ADH is made in the supraoptic nucleus in the hypothalamus, travels down axons and is released into posterior pituitary which distributes it to other tissues.
Secretion is stimulated by an increase in ECF osmolality, volume decrease, NV, angiotensin II and hypoglycemia (so naturally the opposite of these decrease secretion). Acts to increase water uptake by the kidneys --> V2 receptor --> Gs --> increase cAMP --> increase AQP2 channel migration to the collecting ducts |
|
|
How is oxytocin secreted, what prompts its secretion and what are its actions?
|
Oxytocin is made in the paraventricular nucleus in the hypothalamus, travels down axons and is released into the posterior pituitary where it is disseminated to other tissues.
Secretion is stimulated by the Furgusson reflex (pressure on V wall/cervix), suckling, estradiol. Acts to increase milk ejection (suckling), uterine contractions (Furgusson), ovulation (estradiol) Fun fact? I read that the SIGHT or SOUND of a baby can stimulate its release... YIKES! |
|
|
How is prolactin secreted, what prompts its secretion and what are its actions?
|
Prolactin is secreted from lactotrophs in the anterior pituitary when the tonic hypothalamic suppression from dopamine is released.
Binding of prolactin via JAK/STAT pathway to target tissue on breast fosters lactation |
|
|
How is GH secreted, what prompts its secretion and what are its actions?
|
GH is secreted from somatotrophs in the anterior pituitary when it is stimulated by GHRH from the hypothalamus.
Receptors for GH on liver (to make IGF and antagonize insulin action), bone/muscle (directly stimulate linear growth) and fat (promote catabolism and mobilization of TG) |
|
|
What is IGF/IGF1?
|
GH travels to the liver and stimulates the release of IGF which can go and affect everything else that doesn't have a GH receptor on it.
|
|
|
What is the significance of somatostatin with respect to inhibition?
|
Somatostatin inhibits release of GH, prolactin and TSH via Gi decreasing cAMP.
|
|
|
What is Empty Sella Syndrome?
|
Hypopituitarism usually secondary to surgery.
|
|
|
What symptoms are associated with hyperthyroidism? Which symptoms usually are the first to present? What is the number one cause of hyperthyroidism?
|
Symptomatology due to overactivity of the sympathetic nervous system:
1. Cardiac symptoms are the earliest and most consistent --> palpitations, tachy, cardiomegaly, a fib 2. BUG EYESSS do do do do do do do do 3. Neuromuscular- tremor, hyperactive, anxiety 4. Skin- warm, moist, heat intolerance 5. GI- weight loss despite decreased appetite Number one cause is autoimmune (graves) |
|
|
Pt is referred to you with a 2cm anterior pituitary adenoma. Where is the pituitary located and what symptoms would you expect to see associated with the tumor?
|
Pituitary is located in the sella tursica.
With the expansion of a tumor, the optic chiasm can be compressed leading to bitemporal hemianopsia (loss of peripheral field vision). The tumor can also expand and increase intracranial pressure leading to headache, nausea and vomiting. If the tumor is big enough, it can rupture and cause acute hemorrhage called pituitary apoplexy --> DEATH |
|
|
What is Sheehan Syndrome?
|
Post partum necrosis of anterior pituitary d/t ischemia (during pregnancy, the anterior pituitary enlarges to twice its normal size and it not accompanied by an increase in blood supply, so if there is a drastic reduction in blood supply ie. from an obstetric bleed, it can become anoxic and die).
It can also be cause by DIC, increased intracranial pressure, traumatic injury (gunshot wound to the head) |
|
|
How much of the pituitary must be damaged in order to produce symptoms of hypopituitarism?
|
at least 75%
|
|
|
Adenomas in the anterior lobe of the pituitary lead to excess production of hormone. The three most common are Prolactinoma, Somatotroph adenoma and ACTH cell adenoma. What are their symptoms?
|
From most common to least:
Prolactinoma (305)- amenorrhea, infertility, loss of libido Somatotroph adenoma- GH secreting tumor; before puberty? gigantism. after puberty? acromegaly ACTH cell (corticotroph tumor)- Cushings syndrome |
|
|
What is hypothyroidism referred to in adults? Children? Are the symptoms the same?
|
Hypothyroidism in adults: Myxedema- fatigue, cold intolerance, weight gain, reduced cardiac output, SOB, decreased sympathetic activity
Hypothyroidism in children: cretinism- severe mental retardation, short stature, protruding tongue, umbilical hernia; usually as a result of mother's iodine deficiency during pregnancy --> so more common in areas that don't have enough iodine in their diet. **remember, we screen infants for iodine deficiency (T4 values) |
|
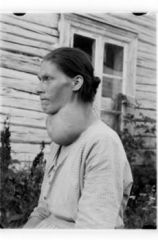
A 24 year old women from the Alps presents to your office with difficulty swallowing and breathing. She looks like this. What happened to her?
|
She has a goiter that is so big, it is compressing her trachea.
Young woman... most likely a diffuse (simple) goiter. Follicles will be normal, just larger and filled with colloid. Impaired synthesis of thyroid hormone due to dietary iodine deficiency. T3/T4 levels would be low to normal, TSH would be elevated. |
|
|
Elderly woman with a history of thyroidectomy presents with muscle pain, episodic tetany, dental problems and a recent change in mental status. You order and EKG and see a prolonged QT interval. What is going on?
|
Hypocalcemia can manifest as tetany due to decreased serum calcium concentration, mental status change, caract formation, CV problems (sp prolonged QT interval). With her history of thryoidectomy, it is not abnormal to have the parathyroids removed too or to have them permanently damaged by the surgery.
She is hypoparathyroid. |
|
|
How would primary and secondary hyperparathryoidism present? What is the number one cause of each?
|
Primary- problem with the parathyroid glands themselves! hypercalcemia (so everything that goes along with that state), increased PTH; usually caused by an adenoma
Secondary: problem further down the line! usually caused by renal failure (can't regulate calcium), hypOcalcemia, increased PTH, symptoms dominated by renal failure problems |
|
|
A 20 year old mentally retarded patient fell on his hand. An xray of th patient shows short 4th and 5th metacarpals with subcutaneous calcification. What syndrome does he have? What is the underlying mechanism?
|
Albright hereditary osteodyrtophy
Form of pseudohypoparathyroidism where the PTH receptor complex is abnormal and there is a loss of responsiveness to PTH. |
|
|
Adrenal gland produces three classes of hormone. Which layers produce which hormones?
What are the names of the three hyperadrenal syndromes produced by hyperactivity of each? |
Zona glomerulosa -->mineralocorticoids --> hyperaldosteroinism (if primary by a solitary lesion-->Conn Syndrome)
Zona fasculata--> glucocorticoids --> Cushings Zona reticularis --> adrogens -->adrenogenital syndrome |
|
|
Ectopic secretion of ACTH is most commonly seen in conjunction with...
|
small cell lung carcinoma
|
|
|
Patient presents with a new onset of episodic heart palpitations, dizziness, chest pain and anxiety. What is the underlying pathology? What tests would you order to confirm your diagnosis?
|
Most likely a Pheochromocytoma- lesion of the adrenal medulla associated with over production of catecholamines.
Rules of 10: 10% in children, 10% bilateral, 10% occur outside of adrenal gland, 10% malignant, 10% are familial (MEN II, III) Order a 24 hour urine metanephrine test |
|
|
What is Cushing syndrome? Most common cause? Other causes?
|
Cushing syndrome is a set of characteristics related to a hypercortisolic state.
Number one cause is exogenous glucocorticoid administration (increased glucocorticoid levels). Also caused by ACTH producing adenoma in pituitary, ectopic secretion of ACTH (SCC). |
|
|
What is Conn Syndrome?
|
Hyperaldosteronism caused by an adrenocortico adenoma
|
|
|
Describe the synthesis and secretion of Thyroid Hormone? What stimulates its synthesis?
|
Thyroid Stimulating Hormone from the anterior pituitary stimulates every aspect of T3/T4 synthesis.
1. TSH stimulates the synthesis of thryoglobulin 2. I- is brought into the follicular cell by iodide symporter and converted into I2 by TPO 3. I2 is organified to thyroglobulin residues by TPO to make MIT and DIT 4. MIT + DIT = T3; DIT + DIT = T4 5. complexes of MIT, DIT, T3, T4 are brought back into the cell where lysosomes cleave off MIT and DIT 6. T3 and T4 are released into circulation bound to throxin binding globulin and go to their target tissues |
|
|
What do thyroid hormones do?
|
4 B's of T3 function
Brain maturation Bone growth Beta- adrenergic effects Basal metabolic rate increase |
|
|
Which enzymes convert T4 to T3? Where can you find this enzyme? What does T3 then do?
|
T4 is converted to active T3 by type 1 and 2 deiodinase (D1, D2)
D1 is found in liver, kidney and thryoid D2 is found in thyroid, skeletal muscle, cardiac muscle, pituitary and hypothalamus. T3 can then cross nucleus, bind to thyroid hormone receptor, cause a bunch of other factors to aggregate (RXR) and increase or decrease gene transcription. |
|
|
What effect does calcitonin have on the body? How does it do this?
|
TONES DOWN CALCIUM
1. inhibits calcium absorbtion in the small intestine 2. inhibits osteoclast activity 3. inhibits phosphate reabsorption in th renal tubules |
|
|
What effect does PTH have on the body? How does it do this?
|
PTH increases serum calcium levels
1. increases osteoblast activity which stimulates osteoclast activity (allows for interaction between RANK and RANKL) 2. increase absorption of Ca from intestine by activating vitamin D 3. enhances resorption of Ca and decreases phosphate resorption from DCT in kidney |
|
|
What type of hormone is PTH? So how is it synthesized?
|
Polypeptide hormone
Synthesized as a preprohormone in cheirf cells which is cleaved to mature PTH and then can be stored to be released when the body needs it |
|
|
Definition of short stature?
|
standing height of more than 2SD below the mean for gender
|
|
|
What imaging modality do radiologists use to determine bone age?
|
AP X ray of the left hand/wrist --> look at the epiphyseal maturation and compare to published standards
|
|
|
Compare bone age and chronological age in:
Familial Short Stature Constitutional Growth Delay Growth Hormone Deficiency Psychosocial Dwarfism |
Familial Short Stature: bone age = chronological age (they are just SHORT!)
Constitutional Growth Delay: bone age < chronological age (they are LATE BLOOMERS, they will catch up eventually) Growth Hormone Deficiency: bone age < chronological age (they will need hormone replacement) Psychosocial Dwarfism: bone age < chronological age (due to nutritional deficiency, broken home, no sleep ect, MOVE THEM SOMEWHERE ELSE AND THEY WILL BE OK!) |
|
|
When is the most human growth hormone released?
|
During Stage III and IV (non-REM) sleep. So sleeping is super important!!!
|
|
|
GH receptors on the liver produce what? What do they do?
|
GH produces IGF --> IGF 1 can then increase the synthesis of cartilage in epiphyseal plates of long bone, increasing bone length
|
|
|
Why do we measure IGF instead of GH to diagnose a GH deficiency?
|
GH levels are pulsatile and have a short half life, so it is difficult to get reliable numbers. IGF is used because GH directly stimulates its production and it has a longer half life. AKA NO WILLY NILLY!
|
|
|
What is the number one cause of GH deficiency?
|
IDIOPATHIC
|
|
|
There are lots of side effects (ohieohsoifheosihleihs) to growth hormone therapy. What is the one we are most worried about?
|
Increased development of malignancy!!!
|
|
|
Most common non-lethal chondrodysplasia? Underlying genetic mutation?
|
Achondroplasia. Mutation in the FGFR gene (fibroblast growth factor receptor)
|
|
|
A mother brings her daughter to you because she is 17 years old and has not started her period (mom started at 12). The girl is only about 4'11''. What do you suspect? What other physical factors would you note?
|
Get a karyotype - TURNERS (XO)
short stature (key to dx, yeah right), webbed neck, increased carrying angle |
|
|
Child comes in for well baby visit. You note a flat, puffy face, large tongue, mental retardation. What is going on? What will bone growth be like?
|
Hypothyroidism- Cretinism
Bone age < chronologic age |
|
|
With Primary, Secondary and Tertiary Growth Hormone deficiency, what structures are affected?
|
Primary- failure of IGF secretion from liver in response to GH
Secondary- pituitary insufficiency Tertiary- defective hypothalamic release of GHRH |
|
|
Somatropin is what type of agent? Clinical use?
|
Recombinant GH
GH deficiency in children (2 or 3), Turner's, end stage renal disease in children, AIDS wasting, malabsorption assd with SBS |
|
|
What are the adverse affects of GH therapy in children?
|
Scoliosis during rapid growth, hypothyroidism (GH increases somatostatin levels which goes back and inhibits thyroid releasing hormone), intracranial hypertension associated visual changes, HA, NV (IGF stimulates CSF formation on the choroid plexus)
|
|
|
What type of drug is Mecasermin? What is it used for? Side effects?
|
Mecasermin is recombinant IGF --> so used for primary GH deficiency
Because it is an IGF analog, it binds up glucose and causes hypoglycemia |
|
|
Octreotide and Lanreotide are what kinds of drugs? What are they used for?
|
Somatostatin analogues --> aka will inhibit GH
Use in acromegaly, gigantism, carcinoid and VIPoma |
|
|
Why is octreotide a good treatment for carcinoid syndrome or VIPoma?
|
Neuroendocrine tumor, such as carcinoid tumors or VIPomas have high expression of somatostatin receptor. Octreotide binds to the somatostatin receptor and inhibits the release of hormones from these tumors.
|
|
|
What are the adverse effects of octreotide and lanreotide?
|
Inhibit GB contraction and decrease bile secretions --> gall stones
Inhibit secretion of insulin and glucagon --> hypoglycemia or hypoglycemia Alter GI absorption of dietary fats --> steatorrhea Octreotide will give you a rrhea tide (**** a lot because it stimulates somatostatins in GI and everywhere else) |
|
|
MOA of Pegvisomant?
|
Pegvisomant is a GH receptor blocker and thus reduces the amount of IGF released, this normalizes IGF in a person with acromegaly
|
|
|
Woman comes because she has not had her period in 8 months. All of her pregnancy tests have been negative but she has noticed a milky discharge from her breasts? What is going on and how would you treat it?
|
PROLACTINOMA!
administer a dopamine agonist (dopamine tonically inhibits secretion of prolactin) like bromocriptine or cabergoline should decrease tumor size and restore ovulation |
|
|
The patient you recently treated for amenhorrhea and milky discharge has come back in because she is suffering from insomnia and when she actually does fall asleep she wakes up with terrible nightmares. What drug did you use for her treatment?
|
amenorrhea and discharge --> prolactinoma
put patient on dopamine agonist like bromocriptine or cabergoline they have severe CNS side effects like psychosis, hallucinations, nightmares and insomnia |
|
|
Pathologies and labs in Primary, Secondary and Tertiary Hypothyroidism?
|
Primary (Hashimotos)- failure of the thyroid to produce adequate thyroid hormone... decreased T3/T4, elevated TSH
Secondary- failure of pituitary to produce TSH in response to TRH from hypothalamus... decreased TSH, decreased T3/T4 Tertiary- hypothalamus is not secreting TRH... decrease TSH, decrease T3/T4 |
|
|
Is the same treatment used for Primary, Secondary and Tertiary Hypothyroidism?
|
YES!! administer T3/T4
|
|
|
Levothyoxine is what type of drug? What is it used for?
|
T4 preparation. Used to treat primary, secondary and tertiary hypothyroidism and TSH suppression in thyroid cancer patients
|
|
|
Normally levothyoxine (T4) is used to treat hypothyroidism. When would you want to use liothyronine (T3)?
|
T4 has a slower, smoother onset and maintenance. You would want to use T3 when you want a faster onset --> myxedema coma!!!!
|
|
|
A woman presented to her family physician with the signs and symptoms of Graves disease. The physician referred her to you, the endocrinologist. How are you going to get her condition under control?
|
Graves Disease is a hyperthryoid disease associated with the production of antibodies to TSH receptor that tonically activates it, stimulating over production of thryoid hormone. So we would want to inhibit the production of thyroid hormone.
Pharmacologically we can use: thoiamides --> inhibit synthesis iodide salts --> inhibit sythesis/release radioactive iodine --> destroy tissue |
|
|
Methimazole and Propythiouracil are what kinds of drugs? MOA? Used in?
|
Thioamides --> inhibit thyroid peroxidase (TPO) from organifying iodine and coupling it with thyroglobulin.
Used in hyperthyroidism to decrease the production of thryoid hormone |
|
|
What are the differences between methimazole and propythiouracil? Which would you use in pregnancy if you had to?
|
Both methimazole and propythiouracil are TPO inhibitors, but propythioruracil also inhibits the peripheral deiodination of T4 to T3
propythiouracil also has a faster onset, but a shorter half life (so better to use in a thyroid storm) Propylthiouracil also more strongly binds to plasma proteins and does not cross the placenta as regularly. |
|
|
What are some side effects of thioamides? And what ones do we have to know again?
|
Methimazole and propythiouracil!
Allergic reaction like effects--> fever, nausea, pruritic rash |
|
|
How does administering iodide salts differ from administering radioactive iodine for the treatment of hyperthrodism?
|
Too much iodine (ie giving iodide salts) it actually inhibits organification to I2 thus inhibiting synthesis of thyroid hormone (wolf- chaikoff effect); this treatment is reversible however
Radioactive iodine destroys the glandular tissue by releasing beta particles; this treatment is not reversible --> can lead to hypothyroidism |
|
|
Should iodide salts be used before or after radioactive iodine?
|
AFTER!!! Why? I still don't understand.
|
|
|
Sodium (Na) is a key component to what song? Is this song good?
|
Rhianna- What's My Name
oh Na Na, what's my name? oh Na Na, what's my name? This song is AWFUL. |
|
|
What percent of adrenal adenomas have large amounts of lipid? How do these adenomas look grossly and on x ray?
|
70% of adenomas have lipid in them; they are homogenous and smoothly marginated.
If you see something with jagged borders that is lumpy, it is not an adenoma. |
|
|
What are the normal dimensions of the adrenal gland?
|
5cm long
5mm thick |
|
|
Best imaging for thyroid? Thyroid nodule?
|
US for both!
|
|
|
How many people will develop thyroid nodules in their life time? Are they benign?
|
5-10% of the population will develop thyroid nodules at some point. Benign.
|
|
|
Image of choice for adrenal gland?
|
CT
|
|
|
Image of choice for pituitary?
|
MRI
|
|
|
Image of choice for pancreas?
|
CT
|
|
|
Image of choice for parathyroid?
|
NUC MED
|
|
|
What three things are black on an MRI?
|
cortical bone, moving blood, air
|
|
|
What color is CSF on a T2 MRI?
What is the difference between a T1 and T2 MRI? |
bright white
T1 is good for anatomy T2 is good for pathology |
|
|
What size is a normal pituitary? Pituitary microadenoma? Macroadenoma?
|
normally is 10 x 10 x 10mm
microadenoma<1cm<macroadenoma |
|
|
What common antidepressant interferes wtih thyroid hormone release into the blood stream?
|
lithium
|
|
|
Elevated TSH signifies thryoid...
|
hypofunction
|
|
|
Decreased TSH signifies thyroid...
|
hyperfunction
|
|
|
Thyroglobulin is what? What is serum thyroglobulin a good measure of?
|
thyroglobulin is aprotein produced only by the thyroid gland
It is used to follow patients after radioactive iodine ablation or surgical thryoidectomy for cancer --> low to undetectable- no recurrence increasing levels- recurrent disease |
|
|
What antibodies are present in Hashimoto's Tyroiditis?
|
anti- TPO
anti- thyroglobulin |
|
|
What antibodies are present in Grave's disease?
|
thyroid stimulating immunoglobulins (aka TSH receptor antibodies)
|
|
|
When would you order an I131 scan? How does it work?
|
When you have suspicion that something is not right AFTER you have gotten an ultrasound.
Iodine is trapped, concentrated and organified by the thryroid gland. By radiographically labeling iodine and measuring the gamma rays emitted we can measure function of the thyroid. |
|
|
How would you interpret a 'hot nodule' on an I131 uptake scan? A 'cold' nodule?
|
hot nodules --> mostly benign
cold nodules --> 5% malignant |
|
|
What is transient hypothyroidism?
What if the patient has a painful presentation? A painless presentation? |
Initial infection with a virus or some sort of inflammatory pathology --> hyperthyroidism due to damage to thyroid follicular cells and unregulated release of preformed thyroid hormones, this lasts 2-6 weekd or until the stores are depleted --> followed by transient hypothyroidism
Painful presentation is typically caused by subacute tranulomatous infection, trauma or radioiodine therapy Painless presentation is usually post partum |
|
|
What is the worst possible presentation of hypothyroidism?
|
Myxedema coma
hypothermia, bradycardia, hypotehsion, multisystem failure must treat agressively with IV thyroxine and external warming to avoid circulatory collapse |
|
|
When treating a patient for hypothyroidism, how long should you wait from when you start treatment to draw blood levels again?
|
6 weeks
|
|
|
What is a non toxic goiter and how should you manage it?
|
A non toxic goiter is an inactive gland that the body is trying to stimulate to make more hormone.
Managed by annual evaluation (palpation, labs, imaging) Levothyroxine to suppress THS and prevent further enlargement Surgery if it is big and compressive |
|
|
Thyroid nodules should have an US to determine if they are cystic or solid.
Solid nodules are more likely... Cystic nodules are more likely... |
solids are more likely to be malignant
cystic are more likely to be benign |
|
|
What are the four types of malignant thyroid nodules from most common to least common?
|
papillary (70-90%, not very aggressive)
follicular medullary anaplastic (very aggressive) |
|
|
What layers make up the adrenal gland? What does each layer produce?
|
Zona glomerulosa- mineralocorticoids
Zona fasiculata- glucocorticoids Zona reticularis- androgens Medulla- catecholamines |
|
|
A deficiency in CYP 21 B hydroxylase would show deficiencies in which hormones? How would this clinically present?
|
Mineralocorticoid deficiency
Glucocorticoid deficiency virilization of women, acceleration of linear growth, early pubic/axillary hair, suppression of gonadal function in men and women |
|
|
A deficiency in CYP 17 would show deficiencies in which hormones? How would this present clinically?
|
Deficiency in Glucocorticoids
Deficiency in Androgens lack of pubic/axillary hair, hypoglycemia, increased ACTH, hypertension |
|
|
How does ACTH stimulate steroidogenesis?
|
ACTH upregulates STAR protein to increase the amount of cholesterol that can be moved into the mitochondria
ACTH also upregulates HSL which breaks down lipid droplets into cholesterol. |
|
|
What is primary adrenal insufficiency? What is deficient? What are the tell tale signs?
|
Addison's Disease
Problem with the adrenal gland itself --> deficiency in all areas --> decreased mineralocorticoids, decreased glucorticoids, decreased androgens, increased ACTH hyperpigmentation, orthostatic hypotension, hypovolemia, hyponatremia, hyperkalemia, hypoglycemia |
|
|
What general classes of drugs would you use to treat Addison's disease?
|
glucocorticoid and mineralocorticoid
|
|
|
What is secondary adrenal insufficency? What is deficient? How can you differentiate it from primary adrenal insufficiency?
|
Secondary adrenal insufficiency is a pituitary problem (decreased ACTH) --> deficient androgens, deficient glucocorticoids, mineralocorticoids are ok because RAAS is still intact
differentiate from primary because there is no pigmentation change in secondary, nor is there any hyperkalemia |
|
|
What general classes of drugs would you use to treat secondary adrenal insufficiency?
|
glucocorticoids only
|
|
|
List the glucocorticoid agents acccording to the least mineralocorticoid activity to the most mineralocorticoid activity.
|
Least mineralocorticoid activity --> dexamethasone --> prednisone -->hydroxycortisone (modest mineralocorticoid activity)
|
|
|
What mineralocorticoid would you use to treat secondary adrenal insufficiency?
|
none. no need for secondary
if primary: fludrocortisone |
|
|
Why is hydrocortisone administered in staggered doses?
|
mimics the normal bodily pulsatile rhythm
highest at 8am and 11pm |
|
|
In primary adrenal insufficiency, both mineralocorticoid and glucocorticoid must be replaced. What mineralocortiocoid should be prescribed?
|
Fludrocortisone
|
|
|
How is the dexamethasone suppression test used to diagnose Cushing's disease?
|
In normal subjects, the administration of dexamthasone results in the suppression of ACTH and cortisol secretion. In Cushing's there is a failure of suppression with low doses, but HIGH doses of dexamethasone will produce suppression from Cushing's (pituitary tumor).
This is not true for an ectopic ACTH secreting tumor. |
|
|
Cushing's syndrome can be caused by...
|
exogenous steroid use
Cushing's disease (pituitary tumor) ectopic ACTH producing tumor adrenal tumor/hyperplasia |
|
|
What is Mitotane and what is it used for?
|
Mitotane is used to kill off the adrenal gland in Cushing's syndrome
exact MOA is unknown, but we think it works like DDT |
|
|
If surgery is not successful in treating Cushing syndrome, you can pharmacologically inhibit adrenal steroid synthesis. How?
|
Aminoglutethiamide
Ketoconazole |
|
|
What is meant by 'determination' and 'differentiation' in adipogenesis?
*Objective* |
Determination is when mesenchymal cells become preadipocytes.
Differentiation is when those preadipocytes become immature multilocular adipocytes. Once they mature, they are mature unilocular adipocytes. |
|
|
What can you use to treat thyroid storm?
|
Propylthiouracil or iodide salts
|
|
|
Secretion of adiponectin is different between lean adipose and obese adipose. What is adiponectin and what is the difference?
|
Adiponectin is a cytokine that increases insulin sensitivity --> the more adiponectin you have, the better off metabolically because it decreases the number of macrophages
Lean adipose prouduces a lot of adiponectin Obese adiponse does not produce a lot of adiponectin |
|
|
What is resisten?
|
increases insulin resistance :(
|
|
|
Excess adipose tissue produces which three adipokines? What do they do?
|
TNF alpha --> increase insuline resistance
IL6 --> increase insulin resistance and impaired glucose tolerance PAI --> increase insuline resistance |
|
|
What is leptin? What does it do to NPY and POMC?
|
Leptin is an appetite suppressant and is secreted in direct proportion to adipose tissue mass
It suppresses appetite by decreasing NPY (appetite stimulant) and increasing POMC (appetite suppressant) |
|
|
Obese individuals produce a lot of leptin (appetite suppressant). So why are they still fatties?
|
There is a ton of leptin bu tthe excess adipose tissue causes it not to work
|
|
|
How do glucocorticoids stimulate adipogenesis and fat deposition?
|
11 beta HSD activates cortisone to cortisol --> cortisol stimulates adipogenesis and fat deposition
|
|
|
Why is excess adipose tissue bad for bones?
|
Adipose tissue produces leptin --> increase sympathetic tone --> increased resorption and decreased proliferation
|
|
|
What effect does serotonin have on bone growth?
|
serotonin secreted by enterochromaffin cells in the gut decreases proliferation and bone formation
Serotonin produced in the brain decreases sympathetic tone and increases proliferation |
|
|
Why do menapausal women have to worry about decreased bone mass?
|
They don't have a lot of estrogen --> increase cytokines --> increase RANK and decrease OPG --> in crease osteoclastic activity
|
|
|
Hyperprolactinemia is often drug related. Which drugs?
|
verapamil (antihypertensive), SSRI (antidepressant) and reglan are most common
*remember young woman with breast discharge on Prozac |
|
|
What is the most common 'functioning' pituitary tumor? What lab values would you see?
|
Prolactinoma
at least 150 ng/ml |
|
|
How is GH treatment administered?
|
SQ injection once/day
|
|
|
What is Kallmann's syndrome?
|
Genetic mutation causing secondary hypogonadism d/t LH deficiency
Often associated with anosmia. *** Classically affects males |
|
|
16 y/o male comes to see you because of delayed puberty. Physical exam reveals very small, firm testicles. Laboratory evaluation reveals testosterone below detectable limits and an LH of 150 (HIGH--RT). Is this primary or secondary hypogonadism?
|
Primary hypogonadism
Klinfelters XXY you had normal before, 150 is high, meaning the pituitary is doing its job, but the target organ isn't responding--RT |
|
|
What percent of the population just walks around with incidental pituitary tumors that you would never know about?
|
10%
|
|
|
If you suspected Cushing's, what test would you order first? Second?
|
24 hour urine for free cortisol
Dexamethasone suppression test second to see if it is from pituitary tumor or ectopic (tumor responds to high dose, ectopic does not respond to any dose) <5 pg/ml in ACTH-independent >20 pg/ml in ACTH-dependent |
|
|
What is the most common world wide cause of primary adrenal insufficiency?
|
TB infection
|
|
|
What is a very common cause of secondary adrenal insufficiency?
|
steroid withdrawl
|
|
|
If you suspect an adrenal insufficiency, what do you always need to rule out before you operate?
|
PHEOCHROMOCYTOMA --> poking and proding can liberate all of those catecholamines --> refractory HTN --> death
|
|
|
Pt presents with episodic dizziness and hypertension. You suspect pheochromocytoma. What test would you order?
|
24 hour metanephrines
Imaging: MRI |
|
|
If you are going to operate on a pheochromocytoma, what must you do prior to surgery?
|
pretreat them with an alpha blocker
|
|
|
What are the differences in plasma renin level in primary and secondary hyperaldosteronism?
|
Primary hyperaldosteronism has low plasma renin
Secondary hyperaldosteronism has high plasma renin |
|
|
What is DHEA? What value sugests adrenal tumor? What value suggests ovarian tumor?
|
DHEA is the principle androgen secreted by the adrenal gland and can undergo further conversion to produce the androgen testosterone and the estrogens estrone and estradiol
over 700 suggests adrenal tumor over 200 suggests ovarian tumor |
|
|
In the US what is the most common cause of Addissons? World wide?
|
US- autoimmune destruction of adrenal
World wide- TB infection |
|
|
How do glucocorticoids act as anti-inflammatory agents?
|
Prevents PLA2 from liberating arachadonic acid
|
|
|
What two things are tell tale for primary adrenal insufficiency?
|
hyperpigmentation
hyperkalemia |
|
|
What is the most common cause of Cushing syndrome?
|
exogenous use of steroids
|
|
|
26 y/o female presents with depression (takes SSRI), increased facial fullness, increased facial hair, irregular menses, weird striae, plethora complexion, buffalo hump. Diagnosis?
|
Cushing's
|
|
|
What is the sympathetic level for the kidneys? Adrenal glands?
|
Kidney- T10-11
Adrenal- T8-10 |
|
|
Where are the chapmans reflexes to the adrenals?
|
anterior is 2'' superior and lateral to umbilicus
posterior is T11 transverse process |
|
|
Where are th chapmans reflexes to the kidneys?
|
anterior is 1'' superior and lateral to the umbilicus
posterior is L1 transverse process |
|
|
When you do thyroid surgery, what structures do you have to look out for?
|
Recurrent laryngeal nerve and parathyroids (have to check calcium levels because of parathyroids)
|
|
|
Whoooooooooo is the best dog in the worldddd?
|
MAC IS
|
|
|
What is bedside rationing?
|
Acting in the best interests of one patient may sometimes make it difficult to act on behalf of another patient who is more likely to benefit from care
Resources, such as time and ICU beds, are limited and people have different priorities for limited resources |

