![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
Formation of prothrombin
|
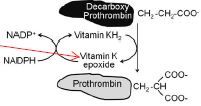
a. Decarboxy prothromin is biologically
inactive unless it is carboxylated. b. The reaction requires carbon dioxide, molecular oxygen, and reduced vitamin K. It is catalyzed by γ-glutamyl carboxylase. c. In the process of carboxylation vitamin K is oxidized to its corresponding epoxide. d. Reduced vitamin K is regenerated from the epoxide by the enzyme vitamin K epoxide reductase. e. In addition to coagulation factor II (prothromin), factors VII, IX, and X and the anticoagulant proteins C and S are biologically activated by the same mechanism. |
|
|
Vessel wall and platelets
|
Platelet membrane receptors
include the glycoprotein (GP) Ia receptor, binding to collagen (C); GP Ib receptor, binding von Willebrand factor (vWF); and GP IIb/IIIa, which binds fibrinogen and other macromolecules. Antiplatelet prostacyclin (PGI2) is released from the endothelium. Aggregating substances released from the degranulating platelet include adenosine diphosphate (ADP), thromboxane A2 (TXA2), and serotonin (5-HT). |
|
|
Fibrinolysis
|
a. Plasmin is generated by the proteolytic cleavage of plasminogen, a
plasma protein that is synthesized in the liver. b. The proteolytic cleavage is catalyzed by tissue plasminogen activator (t-PA), which is synthesized and secreted by the endothelium. c. Plasmin exerts its anticoagulant effect by proteolytically cleaving fibrin into fibrin degradation products. |
|
|
Venous thromboembolism
Formation and risks |
1. Results from clot formation in the venous circulation; manifested as deep
vein thrombosis (DVT) or pulmonary embolism 2. A number of factors increase the risk of developing VTE including age, history of VTE, venous stasis, vascular injury, and drug therapy. |
|
|
Once formed a venous thrombus may either...
|
a. remain asymptomatic
b. spontaneously lyse c. obstruct the venous circulation d. propagate into more proximal veins e. embolize f. act in any combination of these 4. VTE can be debilitating or fatal so it is important to treat it quickly and aggressively. Because the antithrombotic drugs can cause major bleeding, the diagnosis must be made with reasonable certainty. |
|
|
Mechanism of action
Unfractionated heparin (part 1) |
(1) derived from bovine lung or porcine intestinal mucosa
(2) made up of repetitive units of D-glycosamine and uronic acid (3) heparin and LMWH (and fondaparinux below) have no intrinsic anticoagulant activity. Instead, they bind to antithrombin and accelerate the rate at which it inhibits various coagulation proteases (e.g., thrombin and Xa). (4) pentasaccharide on the heparin molecule binds antithrombin provoking a conformational change-the heparin-antithrombin complex is 100x-1000x more potent as an anticoagulant compared to antithrombin alone |
|
|
Mechanism of action
Unfractionated heparin (part 2) |
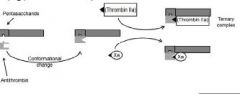
(5) IXa, Xa, XIIa, and thrombin are inhibited; inasmuch as thrombin is
inhibited, the activation of factors V and VIII is inhibited as well (6) the unfractionated heparin molecule contains enough saccharides (>18) to form a ternary complex bridging antithrombin and thrombin inactivating thrombin (7) inactivation of factor Xa does not require the bridging between antithrombin and thrombin; it requires only the binding of antithrombin to the pentasaccharide (8) the anti-Xa to anti-IIa ratio is 1:1 (9) heparin uncouples from antithrombin after it has produced its effect and quickly recouples with another antithrombin (10) the heparin-antithrombin complex is too large to inactivate Xa and thrombin within a formed clot (11) heparin prevents growth and propagation of a formed thrombus allowing the patient’s own thrombolytic system to degrade the clot |
|
|
Enoxaparin
Mechanism |
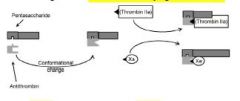
(1) The mechanism is the same as for heparin but it is less effective in
inhibiting thrombin (2) the low-molecular-weight heparins (enoxaparin) have a shorter chain length which limits their activity against thrombin (3) the anti-Xa to anti-IIa ratio is up to 4:1 |
|
|
Pharmacokinetics
a. Heparin |
(1) can be administered subcutaneously or intravenously; the
bioavailability after subcutaneous administration is variable (2) eliminated by two mechanisms (a) heparinases and desulfatases enzymatically inactivate heparin molecules - the process is zero order (saturable) (b) heparin is also eliminated renally (first order) but this process is slower (3) heparin is administered subcutaneously in abdominal fat for prophylaxis or for acute treatment but the intravenous route is preferred for acute treatment (bolus dose followed by continuous infusion) (4) the aPTT is used to assess anticoagulation with heparin; it is determined before administration and 6 hours later or after a dose change |
|
|
Enoxaparin
Pharmacokinetics |
(1) bioavailability and anticoagulant response after subcutaneous
administration is better than with heparin and is the route of administration (2) it is eliminated renally so half-life may be prolonged in patients with renal impairment (3) because the anticoagulant effect is more predictable, routine laboratory monitoring is usually not necessary; when it is, measurement of anti-factor Xa is used |
|
|
Heparin/Enoxaparin
Adverse Effects |
a. Bleeding
(1) can occur at any site (2) when it occurs, heparin should be discontinued immediately and protamine administered; it forms a stable salt with heparin with loss of anticoagulant activity (3) major bleeding with enoxaparin less than with heparin but when it occurs protamine can be use; because the low-molecular-weight heparins have shorter chains, protamine does not bind as well so it cannot completely neutralize anticoagulation b. Heparin-induced thrombocytopenia (1) more commonly seen with heparin (LMWH do not bind platelets and PF-4 to the same degree as heparin) (2) because of cross-reactivity with heparin antibodies, LMWH should not be use in patients diagnosed or have a history of HIT (3) more details are provided below |
|
|
Heparin/Enoxaparin
Drug interactions |
Other anticoagulants can increase the risk of bleeding
|
|
|
Drug that reverses heparins effect
|
Protamine
binds heparin>enoxaparin |
|
|
Fondaparinux
a. Mechanism of action |
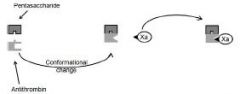
Xa inhib
(1) consists only of the pentasaccharide (2) causes a permanent conformational change in antithrombin (3) it is not destroyed but released to interact with other antithrombin molecules (4) it has no direct effect on thrombin |
|
|
Fondaparinux
Pharmacokinetics/use |
(1) It is rapidly and completely absorbed (100% bioavailabilty) after
subcutaneous administration (2) eliminated renally and thus should not be used in patients with severe renal impairment (3) it is used for prevention and acute treatment of VTE |
|
|
Fondaparinux
Adverse Effects |
bleeding - no specific antidote
|
|
|
Rivaroxaban
a. Mechanism of action |
Direct Factor Xa inhibitor not requiring antithrombin
|
|
|
Rivaroxaban
admin/elim |
(1) Oral administration; rapidly and well-absorbed from the
gastrointestinal tract (2) Eliminated in the urine and is a substrate for P-gp and CYP3A4 (see below Drug Interactions) |
|
|
Rivaroxaban
Adverse effects |
bleeding--no specific antidote
|
|
|
Rivaroxaban
Drug interaxn |
a. Inducers and inhibitors of the transporter P-glycoprotein (P-gp) will have
corresponding effects (decreased and increased, respectively) serum concentrations of rivaroxaban. b. Inducers (e.g., rifampin) and inhibitors (e.g., ketoconazole) of the CYP3A4 will have corresponding effects (decreased and increased, respectively) serum concentrations of rivaroxaban. c. Other anticoagulants (warfarin, heparin, low-molecular weight heparin, anti-thrombin agents) can increase the risk of bleeding d. Anti-platelet drug (aspirin, clopidogrel) can increase the risk of bleeding |
|
|
Rivaroxaban
Clinical Use |
a. Thromboprophylaxis in patients
undergoing hip or knee replacement surgery b. Treatment and secondary prevention of VTE (DVT or pulmonary embolism) |
|
|
Direct thrombin inhibitors
1. Mechanism of action |
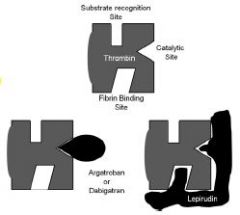
a. Directly interact with the thrombin
molecule b. Able to inhibit circulating and clotbound thrombin c. Lepirudin is the most potent since it binds to both the active site and a second site on the thrombin molecule d. Argatroban and dabigatran bind only to the active site of thrombin |
|
|
Pharmacokinetics
a. Lepirudin |
(1) administered via continuous intravenous infusion or subcutaneously
(2) eliminated renally; adjust dose in patients with impaired renal function (3) monitor therapy using aPTT |
|
|
Pharmacokinetics
Dabigatran |
(1) Dabigatran etexilate is rapidly absorbed from the gastrointestinal
tract and converted by serine esterases to dabigatran, which is the active form (2) Eliminated in the urine; Use with caution in patients with mild to moderate renal impairment, Should not be used in severe renal impairment |
|
|
Pharmacokinetics
Argatroban |
(1) administered via continuous intravenous infusion
(2) eliminated by hydroxylation and aromatization in the liver; reduce dose in patients with impaired liver function (3) monitor therapy using aPTT |
|
|
Direct thrombin inhibitors
(dabig, argatro, lepirudin) Uses |
a. Lepirudin and argatroban - used in patients with HIT to prevent further
thromboembolic complications b. Dabigatran - Primary and secondary prevention of thromboembolism in patients with nonvalvular atrial fibrillation |
|
|
Direct thrombin inhibitors
(dabig, argatro, lepirudin) Adverse Effects |
Bleeding - no specific antidotes
|
|
|
Direct thrombin inhibitors
(dabig, argatro, lepirudin) Drug interaxns |
Other anticoagulants can increase the risk of bleeding
|
|
|
Vitamin K antagonist - Warfain
1. Mechanism of action |
a. The drug inhibits vitamin K epoxide reductase which is the enzyme that
regenerates reduced vitamin K. Warfarin’s action occurs in the liver b. Warfarin has no effect on the activity on the clotting factors that are already formed. Therefore the onset of warfarin activity depends upon the rate of metabolism of the performed factors. (Factor II: 60 hrs, Factor VII: 8 hrs, Factor IX: 24 hrs, Factor X: 40 hrs, Protein C: 14 hrs) c. There is a delay of several days between the drug administration and the peak anticoagulant effect. |
|
|
Vitamin K antagonist - Warfain
admin/elim |
a. Warfarin is well absorbed orally
b. The drug is metabolized in the liver |
|
|
Vitamin K antagonist - Warfain
adverse effects |
a. Bleeding (It can occur at any site and is related to dosage, length of therapy, patient’s underlying disorder and concomitant use of other
drugs.) b. Skin necrosis (Lesions are characterized by widespread thrombosis of the microvasculature and can spread rapidly, sometimes becoming necrotic and requiring debridement or amputation.) c. Fetal toxicity: it includes abortion, fetal bleeding and a characteristic pattern of malformations called fetal warfarin syndrome |
|
|
Vitamin K antagonist - Warfain
drug interaxns: decreased effect |
Drug interactions associated with decreased effect of oral anticoagulants
(shortened PT) (1) Reduced absorption of drug caused by binding to cholestyramine in the gastrointestinal tract (2) Increased metabolic clearance of drug secondary to induction of hepatic enzymes (e.g., barbiturates) (3) Ingestion of large amounts of vitamin K-rich foods or supplements |
|
|
Vitamin K antagonist - Warfain
Contraindications |
a. Any disease that increases the risk of hemorrhage (e.g., aplastic
anemia, leukemia, thrombocytopenia, thrombotic thrombocytopenic purpura, infective endocarditis, inflammatory bowel disease, peptic ulcer, gastrointestinal neoplasms, active pulmonary tuberculosis, etc.) b. Concomitant use of drugs that enhance the anticoagulant effect of warfarin c. Pregnancy |
|
|
Vitamin K antagonist - Warfain
Clinical use |
a. Prevention and treatment of VTE
b. In patients with atrial fibrillation and a presumed cardiac source of embolism, warfarin is the antithrombotic agent of first choice for primary and secondary prevention of ischemic stroke. |
|
|
Vitamin K antagonist - Warfain
Dosing |
a. Genetic variations in the CYP2C9 isozyme (responsible for metabolizing
warfarin) and vitamin K epoxide reductase (VKOR) have been shown to correlate with warfarin dose requirements. b. The dose of warfarin is patient specific based on the desired intensity of anticoagulation and the patient’s individual response. c. There is tremendous intrapatient variability so it is necessary to continually monitor clinical response and laboratory values. d. Routine monitoring of the INR (International Normalized Ratio) is used to assess anticoagulation; the goal range is determined by the therapeutic indication; usually a target of 2.5 with an acceptable range of 2.0-3.0. e. Bleeding or high INR (1) Vitamin K administered orally or intravenously can be used to lower the INR rapidly (2) In cases of serious or life-threatening bleeding, vitamin K should be administered with fresh-frozen plasma, clotting factor concentrates, or recombinant factor VIII. |
|
|
Vitamin K antagonist - Warfain
Drug interactions associated with increased effects |
Drug interactions associated with increased effects of warfarin and thus
hemorrhage (excessive PT prolongation) (1) Decreased metabolism due to P450 inhibition (e.g., cimetidine) (2) Elimination of intestinal flora by antimicrobial agents. Gut bacteria synthesize vitamin K and thus are an important source of this vitamin. (3) Hormone replacement therapy for hypothyroid patients. Hypothyroid patients catabolize coagulation factors more slowly than euthyroid patients. When thyroid function is normalized, anticoagulant effects can increase due to increased rate of catabolism of coagulation factors. |
|
|
Prophylaxis of VTE
|
1. Prophylaxis therapy is given throughout the period of risk
2. Drugs used a. Low-dose heparin subcutaneous administration b. Enoxaparin c. Fondaparinux d. Warfarin e. Rivaroxaban f. Dabigatran is currently indicated only for prevention of stroke and systemic embolism in patients with nonvalvular AF but further studies may establish its value in preventing VTE |
|
|
Treatment of acute venous thromboembolism (DVT or pulmonary embolism)
|
1. Confirm diagnosis
2. Initiate therapy with rapid-acting injectable anticoagulant (heparin, enoxaparin, fondaparinux) a. Stable patients who have low bleeding risk and no other comorbid conditions can be discharged early or treated entirely on an outpatient basis; in these cases, enoxaparin is used b. Rivaroxaban is indicated for treatment of DVT but it does not have the long-term history for use in DVT as the injectable agents have 3. Begin warfarin therapy concurrently with rapid-acting injectable anticoagulant therapy a. Therapy should overlap for at least 5 days and until the INR is $2.0 b. The warfarin dose should be periodically adjusted to maintain an INR between 2.0 and 3.0. c. The minimum duration of warfarin therapy for an acute first episode of VTE is 3 months 4. Rivaroxaban (and probably eventually dabigatran) is an alternative to the use of warfarin and has several advantages including convenience, it does not require routine monitoring. NO antidote for newer drugs |
|
|
Pregnancy
1. Use of anticoagulation therapy for the treatment of DVT or PE in pregnant women |
1. Use of anticoagulation therapy for the treatment of DVT or PE in pregnant
women is common. 2. Low-molecular weight heparins (e.g., enoxaparin) is preferred for prevention and treatment of VTE 3. Fondaparinux and parenteral direct thrombin inhibitors can be used in pregnant patients with severe allergic reactions to heparin (e.g., HIT). 4. Oral direct thrombin inhibitors (dabigatran) and factor Xa inhibitors (rivaroxaban) should be avoided since their effects on the fetus are not fully known. 5. Warfarin should be avoided because it is toxic to the embryo and fetus. Enoxaparin can be substituted |
|
|
Management of heparin-induced thrombocytopenia
1. Pathophysiology |
a. Heparin binds PF4 (released from activated platelets) forming a
negatively charged polysaccharide molecule that is highly antigenic b. IgG antibody production is stimulated and IgG complexes with heparin- PF4 c. The IgG-heparin-PF4 complexes bind to Fc receptor on platelets leading to further activation of platelets and release of PF4 d. PF4 and heparin-like molecules bind to the surface of endothelial cells resulting in antibody-induced endothelial cell damage and the release of tissue factor e. Net result: increased risk of thrombotic events secondary to platelet activation, endothelial damage, and thrombin generation |
|
|
Management of heparin-induced thrombocytopenia
Clinical presentation |
a. Thrombotic complications
(1) VTE is most common including DVT and pulmonary embolism; arterial thrombosis is less common (2) Skin lesions, venous limb gangrene and anaphylactic-type reactions after intravenous heparin are atypical manifestations b. Mortality may be as high as 50% in patients with acute thrombosis |
|
|
Management of heparin-induced thrombocytopenia
Treatment |
a. Goal of therapy is to reduce the risk of thrombosis
b. All sources of heparin, including flushes, should be discontinued c. Direct thrombin inhibitors (argatroban or lepirudin) are the drugs of choice in patients with or without thrombosis d. Patients without thrombosis receive therapy until the platelet count normalizes e. Patients with thrombosis should receive therapy with direct thrombin inhibitors and transition to warfarin after platelet counts recover. Warfarin therapy is then maintained for at least 6 months f. Patients with a history of HIT should receive alternative anticoagulant agents |

