![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
There are two forms of iron available in the diet:
|
(1) heme iron (found in meat, fish & poultry), which has the greatest
bioavailability; and, (2) nonheme iron (found in vegetables & dietary supplements). Absorption of nonheme iron is affected by: (a) phosphates which DECR absorption; and, (b) ascorbic acid and meat which INCR absorption. |
|
|
Iron is best absorbed in its.......(what form)
|
Iron is best absorbed in its ferrous (Fe2+) form, however, dietary iron is in
the ferric (Fe3+) form which is not absorbed. Ferric iron is ionized by stomach acid and then reduced to the ferrous form. The ferrous form is actively taken up primarily in the duodenum but absorptive processes of the mucosa limit the amount of iron absorbed. |
|
|
Internal exchange of iron
|
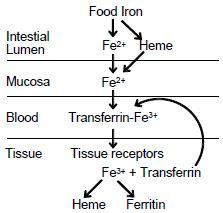
a. Within the mucosal cells, ferrous iron is oxidized to ferric iron which
forms a complex with transferrin, a ß1-glycoprotein with two binding sites for ferric iron. b. This complex binds to specific receptors in the plasma membrane c. and is taken up by receptor-mediated endocytosis. d. Iron dissociates inside the cell and transferrin is returned to the cell surface where it is released to function again. e. If iron is plentiful, then there are fewer transferrin receptors on the surface of cells. This prevents iron-replete cells from receiving excess iron. |
|
|
Storage forms of iron include:
|
1) essential iron-containing
compounds (hemoglobin, myoglobin, enzymes) (2) ferritin (combination of ferric iron with apoferritin) (3) hemosiderin (aggregated ferritin) |
|
|
Sites of iron storage include:
|
(1) the reticuloendothelial system
(2) hepatocytes. Very little iron is lost; about 10%/year in men, more in women due to menstruation. Absorption of iron determines the body’s iron content (see above). |
|
|
Causes of anemia
|
a. Inadequate dietary intake especially during growth in children and in
menstruating and pregnant females b. Malabsorption c. Blood loss |
|
|
Symptoms of iron deficiency anemia
|
a. Feelings of weakness and lassitude
b. Headache c. Dizziness d. Palpitations; chest pain e. Decreased exercise tolerance; shortness of breath |
|
|
Oral iron
|
Ferrous salts (e.g., sulfate) are the treatment of choice.
|
|
|
Absorption of oral Iron, therapeutic effect
|
2) Absorption of ferrous salts is optimal during fasting.
(3) Evaluating therapeutic outcomes (a) hemoglobin recovers in 1–2 months; (b) replenishment of iron stores may require many (3–6) months. |
|
|
Untoward effects of oral iron
|
Untoward effects of oral iron are confined to the gastrointestinal
tract (a) heartburn (b) constipation or diarrhea (c) nausea (more prevalent at higher doses) (d) upper abdominal pain (more prevalent at higher doses) |
|
|
Drug interactions of Oral Iron
|
(a) gastric pH (e.g. antacids, proton-pump inhibitors, H2-
receptor antagonists) --DECR absorption of iron salts (b) Iron can interfere with absorption of many drugs -->Avoid concurrent administration, separate doses by >2 hours. |
|
|
Therapy with parenteral iron
(1) Indications |
(a) Patients in whom GI absorption is prevented by disease
(b) Patient who cannot tolerate orally administered iron (c) Hemodialysis patients (Sodium ferric gluconate and iron sucrose) |
|
|
Therapy with parenteral iron
Iron Dextran info |
i) Administered im or iv
ii) It is a complex of ferric oxyhydroxide with polymerized dextran iii) The complex must be phagocytized by reticuloendothelial cells before iron becomes available iv) Can cause anaphylaxis. Although rare, it can be fatal despite treatment. |
|
|
Therapy with parenteral iron
Sodium Ferric gluconate and iron sucrose |
i) Better tolerated than iron dextran
ii) Delivered to transferrin more readily iii) Lower risk of anaphylactic reactions |
|
|
Iron poisoning
|
Large amounts of ferrous salts by mouth are toxic in adults but
most deaths occur in children between ages 12 and 24 months |
|
|
Iron poisoning
Signs and symptoms |
i) abdominal pain
ii) diarrhea iii) vomiting brown or bloody stomach contents with pills iv) lassitude & drowsiness v) pallor or cyanosis vi) hyperventilation (due to acidosis) vii) cardiovascular collapse viii) Death can occur within 6 hours or be delayed 12-24 hours with a period of apparent recovery in between. |
|
|
Iron poisoning
Treatment |
Treatment includes emesis & lavage with sodium bicarbonate to precipitate iron
|
|
|
Iron poisoning
If plasma concentration of iron > 3.5 mg/L, |
If plasma concentration of iron > 3.5 mg/L, deferoxamine (IM or IV)
is indicated. (a) Deferoxamine has a high affinity for ferric iron. (b) Once bound with iron, feroxamine is excreted in the urine Contraindicated in renal insufficiency and anuria. (c) Deferoxamine can cause allergic reactions (pruritus, wheals, rash, anaphylaxis) |
|
|
Interrelationships and metabolic roles of vitamin B12 and folic acid
|
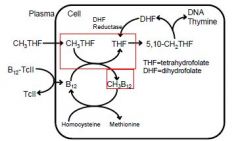
Vitamin B12 and folic acid are dietary essentials for man. Deficiency in either of
these substances results in defective synthesis of DNA in any cell attempting chromosomal repair or division. Tissues with the greatest rate of cell turnover show the most dramatic changes; the hematopoietic system is especially sensitive. Interrelationships and metabolic roles of vitamin B12 and folic acid are shown below. Methyl groups contributed by methyltetrahydrofolate (CH3THF) are used to form methylcobalamin (CH3B12). Thus without vitamin B12, folate is trapped as CH3THF, and subsequent steps in folate metabolism that require THF are deprived of substrate. This provides the common basis for the development of megaloblastic anemia with deficiency of either vitamin B12 or folic acid. |
|
|
Vitamin B12
A. Biochemistry |
Two active coenzyme forms of vitamin B12
a. Methylcobalamin (CH3B12), which is important for folate metabolism and for the formation of methionine and S-adenosylmethionine from homocysteine b. Deoxyadenosylcobalamin which is required for the isomerization of L-methylmalonyl CoA to succinyl CoA. |
|
|
Disposition of vitamin B12
|
1. B12 is released from digested
protein by the actions of gastric acid and pancreatic enzymes 2. Intrinsic factor, secreted from parietal cells binds B12 3. The complex interacts with specific receptors on ileal mucosal cells, is absorbed and enters the circulation 4. Once absorbed, B12 binds with transcobalamin II (TcII) for transport to tissues 5. The complex is preferentially distributed to the liver (up to 90%) 6. Most circulating cobalamin is bound to transcobalamin I and III 7. An alternate pathway for absorption exists which does not require intrinsic factor or an intact terminal ileum. The pathway involves passive diffusion and accounts for 1% of ingested B12. |
|
|
B12 Deficiency--Causes
|
Deficiency (takes several years to develop following vitamin deprivation du eot
enterohepatic circulation of the vitamin) 1. Causes a. Intestinal disease or defects that interfere with absorption of intrinsic factor-B12 complex b. Inadequate secretion of intrinsic factor (pernicious anemia) c. Damage to ileal mucosal cells by disease or surgery |
|
|
B12
Effects of deficiency |
a. Megaloblastic anemia
b. Potentially irreversible nerve damage causing a wide range of neurological signs and symptoms including parethesias of the hands and feet, decreased vibration and position senses with resultant unsteadiness, decreased deep tendon reflexes, and, in later stages, confusion, moodiness, loss of memory, and loss of central vision |
|
|
B12 Deficiency Diagnosis
|
a. Determination of the plasma concentration of B12
b. Examination of blood smear (macrocytosis and hypersegmented polymorphonuclear leukocytes) |
|
|
B12 Deficiency--Treatment
|
a. Treatment includes parenteral (IM or SC) cyanocobalamin, especially in
patients with pernicious anemia or ileal disease b. Oral cyanocobalamin (In patients with pernicious anemia high doses are used since cobalamin is absorbed via passive diffusion) c. Nasal spray d. Evaluating therapeutic outcomes (1) Patient experiences improved strength and well-being within days (2) Plasma becomes normoblastic after 7 days (3) Neuropsychiatric signs and symptoms can be reversible if they are < 6 months’ duration but 6 months of replacement therapy or longer may be necessary for improvement. If permanent neurologic damage has resulted, progression will cease with replacement therapy. e. Once begun, therapy is lifelong. |
|
|
Folic acid
A. Biochemistry |
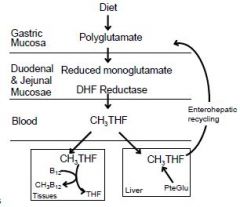
Pteroylglutamic acid (folic acid) is
reduced to tetrahydrofolic acid which acts as an acceptor of one-carbon units |
|
|
Folic acid
Disposition |
1. Polyglutamates in food are
hydrolyzed, reduced, & then methylated by dihydrofolate reductase 2. methyl-THF is transported to tissues and is taken up by receptor-mediated endocytosis 3. Methyl-THF acts as a methyl donor (for formation of CH3B12) and as a source of THF. 4. The liver reduces & methylates folic acid (PteGlu) and transports CH3THF into the bile for reabsorption by the gut. 5. Folate is stored within cells as polyglutamates. |
|
|
Folic Acid
Deficiency Causes |
Causes of deficiency (4-5 months for megaloblastosis to develop once
dietary folic acid is stopped) a. Acute or chronic alcoholism (most common) where daily intake is DECR & ethanol is toxic to hepatocytes b. Drugs (1) Inhibitors of dihydrofolate reductase (methotrexate, trimethoprim) (2) Drugs which interfere with absorption and storage of folate (certain anticonvulsants and oral contraceptives) c. Diseases of small intestine which interfere with absorption of folate & enterohepatic recycling. |
|
|
Folic acid---Deficiency Effect
|
The principal effect of folic acid deficiency is:
a. Megaloblastic anemia b. Unlike B12 deficiency, neurological abnormalities are NOT common |
|
|
Folic Acid--Deficiency Dx
|
Diagnosis
a. Determining plasma concentration of folate b. Examination of blood smear |
|
|
Folic Acid--Treatment
|
a. Therapy is indicated for
(1) Megaloblastic anemia which is treated with IM B12 and folic acid. (2) Folate also is used for prophylactic administration when there is increased utilization such as in pregnancy and lactation. (Folate deficiency during pregnancy is associated with congenital neural tube defects.) b. Preparations (1) Oral and parenteral preparations of folic acid; and (2) Leucovorin (folinic acid) which circumvents the action of inhibitors of dihydrofolate reductase and potentiates the cytotoxic effects of 5- fluorouracil. c. Evaluating therapeutic outcomes Megaloblastic erythropoiesis disappears within 48 hours d. Drug interaction: High doses counteract the effects of antiepileptic drugs. |

