![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
97 Cards in this Set
- Front
- Back
|
What are the categories of drugs that act predominantly in the proximal convoluted tubule?
|
C.A. inhibitors
Osmotic diuretics Loop diuretics |
|
|
Name a C.A. inhibitor:
|
Acetazolamide
|
|
|
Name an osmotic diuretic:
|
Mannitol
|
|
|
Name the three LOOP DIURETICS:
|
Furosemide
Bumentanide Torsemide |
|
|
Name eight diuretics that act predominantly in the distal convoluted tubule:
|
Hydrochlorothiazide
Metolazone Spironolactone Amiloride Chlorthalidone Eplrenerone Triamterine |
|
|
Name two drugs that act on collecting ducts by V2R agonism:
|
Arginine vasopressin
Desmopressin (DDAVP) |
|
|
Name two drugs that act on collecting ducts by V2R antagonism:
|
Conivaptan
Tolvaptan |
|
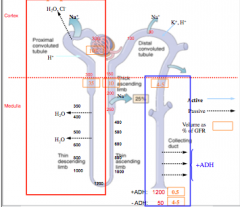
Identify where AQP-1 and AQP-2 is located.
What does this indicate? |
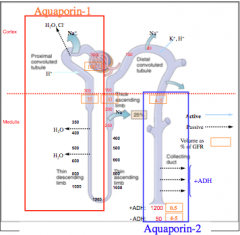
Only places that are permeable to water.
|
|
|
Carbonic anhydrase inhibitors:
Mechanism of action. Drug |
Inhibit C.A. --> bicarbonate loss in the urine
Acetazolamide |
|
|
CA Inhibitor:
Net effect |
Alkaline urine (from Na-bicarbonate loss in urine)
Enhanced chloride reabsorption --> acidosis |
|
|
CA inhibitor:
Clinical uses |
Diuretics: limited use
Alkalinize urine (Cysteinurea) Reduce intra ocular pressure Seizure management Prophylaxis for mountain sickness |
|
|
Side effects of CA inhibitors:
|
Metabolic acidosis
Greatly increased K+ loss in urine (acute effect) |
|
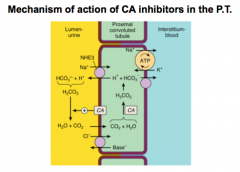
Review this slide and think about how CA inhibitors work.
What happens to pH of urine in carbonate loss? What happens when more chloride is reabsorbed? |
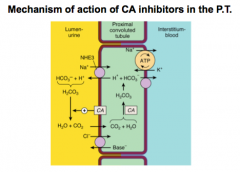
Alkalinizes the urine.
For Cl- think HCl = acidosis |
|
|
What are the characteristics of osmotic diuretics?
|
Small molecules that are filtered, but not reabsorbed by the kidney.
|
|
|
What is the minor mechanism of action of osmotic diuretics?
|
Osmotically inhibit Na+ and H20 reabsorption
|
|
|
What is the major mechanism of action of osmotic diuretics?
|
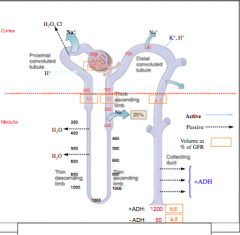
Large doses --> increase plasma osmolarity
1. Extract water from peripheral tissues and decrease blood viscosity 2. increase in medullary renal blood flow and reduce its tonicity. 3. Impair water reabsorption by thin descending limb of Henle's loop (depends on tonicity difference). 4. Impair NaCl and urea extraction by THIN ASCENDING limb of Henle's loop. 5. Interfere with transport processes in the TALH. |
|
|
What is the net effect of osmotic diuretics?
Name a drug. |
Significantly increase urine with small increments in NaCl and other ions.
Mannitol (injection) = ONLY DRUG TO KNOW FOR OSMOTIC DIURETICS |
|
|
When are osmotic diuretics used?
|
Treatment of dialysis disequilibrium syndrome
Reduce intracranial pressure (from severe head trauma) Reduce intraocular pressure |
|
|
What is a side effect of osmotic diuretics? What patients are they contraindicated in?
|
Volume overload
Contraindicated in cardiac failure. |
|
|
What is the MOA of loop diuretics?
What does this causes (macula densa)? What is stimulated? What is increased? What is maintained? Why are they called "high ceiling?" |
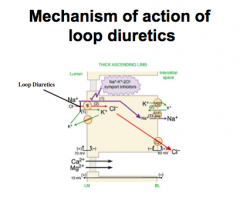
Inhibit Na-K-2Cl symporter in TALH (K+ must seep out).
Inhibit the ability of macula densa to "sense" NaCl. Stimulated biosynthesis of prostaglandins. Increase in total renal blood flow. Maintain GFR. Loss of sodium and water in urine due to inhibition of TALH Na+ reabsorption |
|
|
How do loop diuretics potently increase renin release?
|
1. Inhibition of macula densa
2. Reflexively activate sympathetic NS 3. Stimulation of intrarenal baroreceptor mechanisms. |
|
|
What are the 5 net effects of loop diuretics (high-ceiling diuretics)?
|
1. Mobilize NaCl (most potent class of diuretics that does this).
2. Copious diuresis and significant NaCl loss. 3. Increase urinary excretion of K+/H+ 4. Increase excretion of Ca2+ and Mg2+ 5. Impair ability of kidney to concentrate urine. |
|
|
What four clinical situations require the use of loop diuretics?
|
1. Edema (cardia, hepatic, or renal origina)
2. Pulmonary edema 3. Hypercalcemia (to mobilize Ca2+) 4. Protect against renal failure |
|
|
How do loop diuretics help with pulmonary edema?
|
1. Decrease pulmonary wedge pressure
2. Increase compliance of pulmonary vessels 3. Increase peripheral vascular capacitance 4. Reduce left ventricular filling pressure 5. Cause brisk diuresis |
|
|
What is one specific example of a loop diuretic (the common one)? Where does it act? How is it administered? What is it secreted by?
|
Furosemide
Sulfonamide drug that inhibits NaCl reabsorption in TALH (oral and IV/IM) Secreted by organic acid transporter to inhibit luminal symporter. |
|
|
True or false. Furosemide has a wide margin of safety.
Describe the dose response curve of furosemide that is influenced by renal disease. Why? |
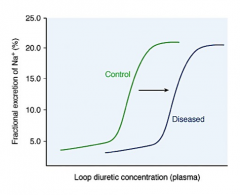
True.
Due to impaired secretion of the drug. |
|
|
What are the five pharmacological effects of furosemide?
|
1. Copious diuresis with significant NaCl losses.
2. Increased urinary excretion of K+/H+ 3. Increased urinary excretion of Ca2+ and Mg2+ 4. Increased renal prostaglandins 5. Increased venous capacitance |
|
|
How is furosemide administered?
How long until a diuretic response? How long does it last? What is the half life? What is the drug bound to? How much of drug is excreted in urine? |
Orally OD or BID
30 minutes until response --> lasts 8 hours 1.5 hours (short), extensively protein bound 65% excreted in urine HIGH MARGIN OF SAFETY! |
|
|
What are the 3 major side effects of furosemide?
|
1. Fluid/electrolyte abnormalities (hypokalemia and alkalosis) --> requires initial monitoring
2. Elevated BUN, hyperglycemia, hyperuricemia 3. Ototoxicity, sialadentitis (inflammation of salivary glands) |
|
|
What are common drug interactions of furosemide?
|
1. Lithium
2. Indomethacin 3. Probenecid 4. Warfarin |
|
|
Which loop diuretic is forty times more potent than furosemide? Why would you ever use it?
|
Bumentanide --> use instead of furosemide if patients are receiving warfarin
|
|
|
Which loop diuretic also lowers BP? Anything special about its half life?
|
Torsemide --> much longer half-life than other loop diuretics, so just given once daily.
|
|
|
What are the three big segments of the distal convoluted tubule and collecting ducts?
|
1. Na+-K+ aldosterone independent segment (Na-Cl symporter).
2. Aldosterone sensitive segment. 3. Sodium load segment. |
|
|
What two types of Na+ channels are expressed in IMCD?
|
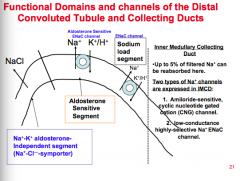
1. Amilioride sensitive cyclic nucleotide gated cation (CNG) channel.
2. Low conductance highly selective Na+ ENaC channel. |
|
|
What is the mechanism of action thiazide diuretics?
BENZOTHIADIAZIDES |
Inhibit NaCl reabsorption in the Na-K aldosterone independent segment of the distal tubule.
|
|
|
What are the six pharmacologic effects of thiazide diuretics?
Big difference between thiazide and loop** |
1. Moderate loss of Na+, K+, and Cl- and causes a 3X increase in urine flow.
2. Serum loss --> reduced GFR (chronic) 3. Elevation of urinary potassium (hypokalemia) 4. Increased delivery of Na+ to distal tubule --> increased excretion of titratable acid. 5. Decrease urinary excretion of Ca2+.** 6. Increase urinary excretion of Mg2+. |
|
|
What are the three important uses of thiazide diuretic?
|
1. Diuretic = reduce edema with CHF, cirrhosis, and nephrotic syndrome
2. Hypercalciurea and renal calcium stones. 3. Nephrogenic diabetes insipidus. 4. Osteoporosis (think calcium). |
|
|
When are most thiazides ineffective (GFR)?
Do thiazides require excretion into tubular fluid to exert effect like loop diuretics? |
GFR < 30-40 mL/min except for metolazone or indapamide
Yes! |
|
|
What are the two classes of thiazide diuretics? When do you use which?
|
Class 1 = if GFR > 50 mL/min
Class 2 = more potent, use if GFR >30 and <50 |
|
|
What are three examples of class I diuretics?
Which has the shortest half life? |
Hydrochlorothiazide (2.5 hour half life)
Chlorthalidone Quinethazone |
|
|
What are two examples of class II thiazides? Which is the most potent?
|
Metolazone (10x more potent than HCTZ)
Indapamide (20X more potent than HCTZ) |
|
|
What are four adverse reactions of thiazides?
|
Deletion phenomena
Retention phenomena Metabolic changes Hypersensitivity and others |
|
|
Examples of the deletion phenomena?
|
HyPOkalemia, hyPOchloremic alkalosis, hyPOmagnesemia, dilutional hyPOnatremia
|
|
|
What are two examples of the retention phenomena?
|
hyPERuricemia
hyPERcalcemia |
|
|
What are three examples of metabolic changes?
|
hyPERglycemia
hyPERlipidemia hyPERsecretion of renin and aldosterone |
|
|
What are some "hypersensitivity and other" reactions?
|
Fever, rash, purpura
Pancreatitis Sialadentitis Withdrawal edema |
|
|
Between thiazides and loop diuretics, which has the higher NaCl excreted per dose?
|
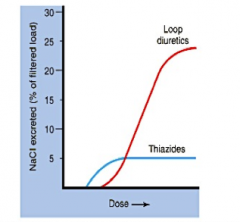
|
|
|
What two types of cells are in the late DCT and CD?
What are the two principal cell types? What is each involved in? How is each regulated? What happens when Na+ channel is inhibited? |
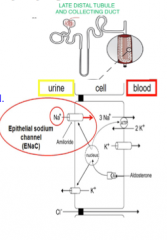
Principal and intercalated cells
Type A (Na+ reabsorption, K+ secretion --> hormonally regulated by aldosterone) Type B (Na + reabsorption, K+ secretion --> load dependent regulation --> the more Na+ delivered, the more absorbed in exchange for K secretion) Increase Na+ excretion, reduce K+ secretion. |
|
|
What is the physiological action of aldosterone (pathway)?
|
Binds to receptor in DCT --> moves to nucleus, binds to HORMONE sensitive elements --> regulation of aldosterone-incuded EnaC Na+ channels --> enhanced transepithelial NaCl transport --> increase of lumen-negative transepithelial voltage --> increase in K+ secretion and H+ secretion.
|
|
|
What is the mechanism of action of aldosterone antagonists?
|
1. Bind aldosterone receptor in cytoplasm and prevents translocation to nucleus.
2. Reduce Enac channels that are involved in Na+ reabsorption. |
|
|
What is the net effect of aldosterone antagonists?
|
1. Increase urinary excretion of Na+ (NATRIURETIC)
2. Inhibition of secretion of K+ and H+ (K-sparring) |
|
|
Give an example of an aldosterone antagonist.
Is this a prodrug that is extensively metabolized? Which drug is an active metabolite with a longer half-life? |
Spironolactone. Yes
Canrenone |
|
|
What are the side effects of aldosterone antagonists?
How do you protect against hyperkalemia? |
Hyperkalemia (combine with thiazide)
Gynecomastia, hirsutism, uterine bleeding |
|
|
What are the clinical uses of aldosterone antagonists?
|
Diuretics (in combination with HCTZ)
Used to treat CHF and cirrhosis. |
|
|
What are some examples of organic bases not structurally related to aldosterone (K-sparing diuretics)?
|
Triamterene, amiloride
|
|
|
What is their mechanism of action?
|
Inhibit Na reabsorption in late distal tubule (sodium load segment).
|
|
|
What are the pharmacological effects of triamterene and amiloride (k-sparing diuretics)?
|
Increase urinary excretion of Na+ (weak effect)
Inhibit secretion of K+ and H+ (K-sparing) |
|
|
What are the clinical uses of K-sparing diuretics?
Side effects? |
Diuretics --> combinted with HCTZ to increase effectiveness and decrease K+ excretion
Hyperkalemia Megaloblastic anemia in patients with cirrhosis |
|
|
What are the four types of atrial natriuretic peptide? Where is each found? Response of each?
|
ANP, BNP = produced by heart in response to stretch
CNP = endothelial and renal origin Urodilantin = found in urine, paracrine regulator of Na+ transport |
|
|
MOA of ANP?
MOA of BNP? |
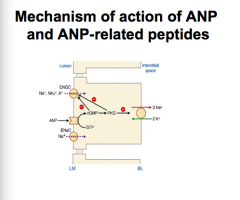
ANP = atrial peptide produced in response to stretch --> binds NP receptor A (NPR) --> GC activated --> increase cGMP
BNP = produced by ventricle --> binds NPR-A similar to ANP |
|
|
MOA of CNP?
|
Binds NPR-B in vascular smooth muscle cells and mediates relaxation.
|
|
|
MOA of urodilatin?
|
Arises from same precursor of ANP. Binds with low affinity to ANP-B in the glomeruli and IMCD.
|
|
|
What is an example urodilatin? What are its clinical effects?
|
Nesiritide
Increases Na+ excretion by inhibiting CNG nonspecific cation channel in IMCD and by inhibiting the renin angiotensin system and endothelial production. |
|
|
Why is nesiritide usefeul in CHF? Three things.
|
1. Decreases systemic vascular resistance
2. Decreases left ventricular filling pressure 3. Increases cardiac output. (no data that it actually reduces mortality though) |
|
|
What are the side effects of nesiritide?
|
Numerous due to narrow therapeutic window
|
|
|
What are the best tolerated drugs for the monotherapy of hypertension? Which have been demonstrated to decrease mortality in hypertension patients? Responses to diuretics are seen at lower or higher doses?
|
Diuretics and ACEIs
Diuretics (alone or in combo with beta blockers) Low = should avoid high due to side effects |
|
|
What are thiazides mostly used for? What is the most popular drug for therapy of high BP? Which drugs are effective in patients with impaired renal function when thiazides are not? When are these drugs ineffective?
|
Mild-->moderate HT
HCTZ Metolazone and indapamide (not effective when GFR <30 or creatinine > 2.5 mg/dL |
|
|
Which type of HT patients show a better response to thiazides? What would a poor response reflect?
What is the best way to administer HCTZ? |
"Volume dependent" HT (low renin levels)
High load of dietary sodium or impaired renal capacity to excrete sodium Single morning dose (sustained effect, reduces K+ wastage during nighttime). |
|
|
When are loop diuretics used? Is more frequent monitoring required? How are these diuretics administered and when?
|
Severe HT unresponsive to thiazides, renal insufficiency, cardiac failure, cirrhosis
Yes = can have more serious side effects IV in HT crisis or acute pulmonary edema |
|
|
Administration of what is a common preventable causes of diuretic resistance. Why?
|
NSAID
Prostaglandins --> reduce Na+ reabsorption, antagonist ADH, and distribute renal blood from cortex to the juxtaglomerulus |
|
|
What is useful to use in patients at risk of K+ depletion or in patients with hyperuricemia? What is the drug of choice in cirrhosis? What do you do if GFR is less than 50 mL/min? When are they contraindicated?
|
K+ sparing diuretic
Spironolactone (add a loop diuretic) Significant renal insufficiency (GFR < 75) or other K+ retaining conditions |
|
|
Where is ADH synthesized?
|
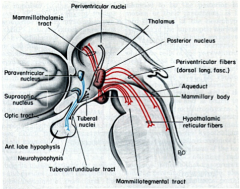
Paraventricular and supraoptic nuclei of hypothalamus
|
|
|
When is ADH released?
|
1. Plasma osmolarity > 280 mOsm/Kg
2. Depletion of extracellular volume 3. Pain, nausea, hypoxia |
|
|
Where is the V1 receptor found (ADH receptor)? What is the result of binding?
|
Vascular smooth muscle
Activation of Gq-PLC-IP3 pathway --> mobilization of Ca2+ --> vasoconstriction |
|
|
Where is the V2 receptor located? What happens upon binding?
|
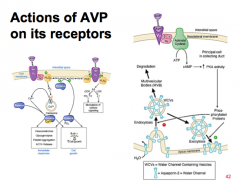
Principal cells in renal collecting ducts
Gs-cAMP, PKA activated --> increase rate of insertion of water channel containing vesicles (WCVs) into the apical membrane of CD. PKA --> phosphorylates AQP-2 --> inserted as tetramers into apical membrane --> increase permeability of CD to water. PKA --> phosphorylates urea transporter (VRUT or UT1) --> increased permeability of CD to urea. |
|
|
What is the net effect of ADH?
|
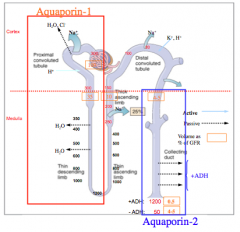
Concentrates urine up to four times the osmolarity of plasma.
|
|
|
What are aquaretics? What does activation of V1R cause?
|
Synthetic peptides that are V1 receptor antagonists (ANTI-DIURETICS).
Activation of V1R --> GI and vascular smooth muscle contraction. |
|
|
What are the clinical uses of V1R agonists?
|
IV to treat:
Post oprative ileus Reduce bleeding in esophageal varices Reduce bleeding during acute hemorrhagic gastritis |
|
|
What are examples of aquaretics?
|
8-arginine vasopressin (discontinued due to side effects)
Terlipressin (fewer side effects) |
|
|
What are the two types of diabetes insipidus? Symptoms?
|
1. Inadequate AVP secretion (central DI)
2. Insufficient kidney AVP response (nephrogenic DI) Symptoms: excrete large amounts of urine, drink a lot of water (polydipsia) |
|
|
How do you distinguish central DI from nephrogenic DI?
|
Administration of V2R agonist (desmopressin) --> will increase urine osmolarity in patients with central DI but no nephrogenic DI
|
|
|
What are some causes of central DI?
|
Acquired:
Head trauma, surgery or trauma in pituitary or hypothalamus, tumors, CNS ischemia (think head problems) Genetic: Autosomal dominant (chrom 20) --> loss of AVP |
|
|
How do you treat central DI?
|
Synthetic vasopressin peptides: selective V2R agonists
|
|
|
How is DDAVP (desmopressin) administered? What is it also used in?
|
Nasally, IV, or oral.
Bleeding disorders, nocturnal enurisis (bed wetting in children) |
|
|
What are some causes of nephrogenic DI?
|
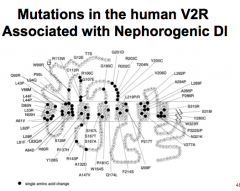
Acquired:
Obstructive renal disease, drugs: Li, clozapine Genetic: X-linked gene encoding V2R --> frameshift, truncated receptor, or single amino acid mutation |
|
|
How do you treat nephrogenic DI?
|
Maintain adequate water intake
Thiazide diuretics (may reduce polyuria by 50%) --mild depletion of EC water and sodium --> compensatory mechanisms increase reabsorbative capacities of PCT --> reduce volume delivered to distal |
|
|
What is SIADH?
|
Too much ADH produced --> impaired water excretion and plasma hypoosmolarity (HYPONATREMIA)
|
|
|
What are the three drug classes associated with SIADH?
|
Psychotropics (SSRI, haloperidol, tricyclic antidepressants)
Sulfonylureas: chloropropamide Vinca alkaloids: vincristine, vinblastine |
|
|
What do you use to treat SIADH and hyponatremia?
|
1. Water restriction, IV hypertonic saline
2. Loop diuretics 3. Demeclocycline: antagonizes ADH at V2 receptors 4. Vaptans |
|
|
What are some other water-retaining conditions? Pathways?
|
CHF, cirrhosis, nephrotic syndrome --> reduction of effective blood volume --> hypovolemia exacerbated by diuretics --> AVP release --> hyponatremia
|
|
|
What are vaptans? Give an example? Why is it a good choice for treatment of SIADH and hyponatremia?
|
Selective V2R antagonist
Tolvaptan 29X more selective for V2R than V1aR |
|
|
What are the indications for tolvaptan? When is it used (hospital or at home)?
|

SIGNIFICANT hypervolumemic and euvolemic hyponatremia (less than 15 mEq/L)
Symptomatic hyponatremia that resists correction with fluid retention USED ONLY IN A HOSPITAL SETTING!! |
|
|
What is the metabolism of Tolvaptan (cyp?)
Side effects? |
CYP3
Hyperglycemia, GI disturbances, and clotting problems |
|
|
Which V2R antagonist is less selective than Tolvaptan? How is it given? Metabolism? Side effects?
|
Conivaptan = IV for acute treatment of hyponatremia in hospital setting.
Metabolized by CYP3A4 Numerous = infusion site reaction |
|
|
What are the two types of aquaretics?
|

V1a selective antagonist
V1b selective antagonist |
|
|
Where are the nephron sites of action of diuretics?
|
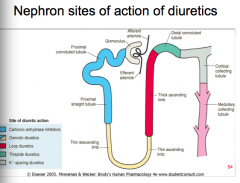
|
|
|
Look at review slides in lecture.
|
Look at review slides in lecture.
|

