![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
|
White Blood Cellll Countt (WCC)
|
White Blood Cellll Countt (WCC)
Full Blood Counts will provide information on total White Cells This is total of all components of White Cells Differential usually included which breaks total WCC into these different elements Elevation - most commonly infection of some type, although may rise as acute phase reaction eg inflammation, tissue damage |
|
|
WCC relative Differential
|
WCC relative Differential
Total WCC (leucocytes) 5-14 x 103 cells/mm3 Segmented Neutrophils (PMNs) 60% Band Neutrophils 2% Lymphocytes 25% Monocytes 8% Eosinophils 3% Basophils 2% |
|
|
overally picture of wcc formation
|
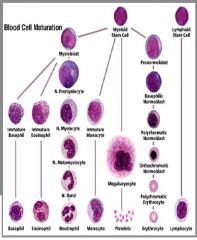
|
|
|
Leukocytes - categories and characteristics
|
Leukocytes
|
|
|
Phagocytes - types
|
Phagocytes
• Phagocytes include granulocytes (neutrophils, eosinophils, basophils) and monocyte macrophages. Neutrophils are further divided into polymorphonuclear leukocytes and band leukocytes. Granulocytes |
|
|
Phagocytes- characteristics
|
Phagocytes
Phagocytes possess no memory i.e. has no mechanism for increasing response on future exposure Phagocytes have receptors for carbohydrates that are not normally expressed in vertebrates, they also receptors for antibodies and complements. Therefore, they can distinguish between self and non-self. Phagocytes also remove the bodies dead and dying cells. Dying cells in necrotic tissue release necrotic substances that trigger an inflammatory response Dying cells from programmed cell death (apoptosis) express molecules on their cell surface that identify them as candidates for phagocytosis |
|
|
Neutrophils
|
Neutrophils
They are the most populous circulating white cells (60%). They are produced in response to stress: Infection, trauma, emotional distress, or other noxious stimuli. (*A each pack of cigarettes per day can raise the count by 1000/μL.) Neutrophils phagocytose “invaders” but kill themselves in the process. |
|
|
Shift to the Left
|
Shift to the Left
Total WCC 5-14 x 103 cells/mm3 Segmented Neutrophils (PMNs) 60% Band Neutrophils 2% Lymphocytes 25% Monocytes 8% Eosinophils 3% Basophils 2% Segs \ Lympo \ Mono \ Eosino \ Baso \ Bands |
|
|
Monocytes - what do they turn into, what are they good and bad at
|
Monocytes
Monocytes are precursors to Macrophages Monocytes circulate in the blood - activated to macrophages in the tissue Macrophages in the tissue present antigens to lymphocytes. Macrophages are good at phagocytosis and poor at killing. Some micorganisms can survive in macrophages for years. |
|
|
Eosinophils
|
Eosinophils
? No one really is sure what they do. They often seen at the site of invasive parasitic infections i.e. counts are raised. Individuals with chronic allergic conditions (atopic rhinitis or extrinsic asthma) typically have elevated eosonophil counts |
|
|
Basophils
|
Basophils
? No one really is sure what they do. ⇑ in chronic inflammation ⇑ in leukaemia Same as mast cell?? In allergic conditions blood basophils decrease in number while tissue mast cells increase Basophils contain heparin, histamine, and leukotrienes |
|
|
Lymphocyte
|
Lymphocytes
• Lymphocytes: are the other components of WBC (25%). Lymphocytes are brainy- These cells give specificity and memory to the body’s defense against invaders. They have two categories: B-Lymphocytes T-Lymphocytes Phagocytes are dumb |
|
|
B-Lymphocytes
|
B-Lymphocytes
• B-lymphocytes are derived from the bone marrow i.e. B-lymphocyte. B-lymphocytes development is regulated by T lymphocytes B-lymphocytes are involved in humeral immunity. (humeral immunity; antibody immunity). B-lymphocytes develop into plasmocytes that produce antibodies. |
|
|
over view of all wcc
|
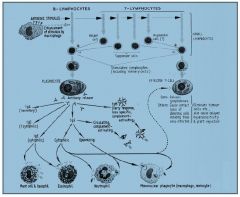
|
|
|
T-Lymphocytes
|
T-Lymphocytes
• T-lymphocytes are derived from the bone marrow but they mature in the thymus i.e. T-lymphocyte. T lymphocytes regulate B-lymphocytes development T-lymphocytes are involved in cellular immunity and T killer cells. Responsible for delayed hypersensitivity, rejection of organs and defense against viral infections |
|
|
Neutrophilia - level and cause
|
Neutrophilia > 12,000 cell/mm3
– Acute bacterial infection – Trauma – Myocardial infarction – Chronic bacterial infection – Adrenaline, corticosteroids, lithium – Leukaemia |
|
|
Neutropenia - level cause
|
Neutropenia < 1,500 cell/mm3
– Radiation Exposure – Medications: cytotoxics, captopril, ticlopidine etc – Overwhelming acute bacterial infection – Vitamin B12 or folate deficiency – Salmonellosis – Pertussis |
|
|
Eosinophilia and Eosinopenia
|
Eosinophilia > 350 cell/mm3
• Causes include – Allergic disorders (asthma) – Parasitic infections – Leukaemia – Medications: ACE inhibitors, allergic reactions to drugs Eosinopenia < 50 cell/mm3 •Causes include •Acute infection |
|
|
Basophilia
|
Basophilia > 300 cell/mm3
• Causes include – Chronic inflammation – Leukaemia Monocytosis > 800 cell/mm3 • Causes include •Recovery stage of acute bacterial infection •TB disseminated •Endocarditis •Protozoal infection •Leukaemia |
|
|
Lymphocytosis causes
|
Lymphocytosis > 4,000 cell/mm3
• Causes include – Infections •Lymphopenia < 1,000 cell/mm3 Causes include •HIV •Radiation exposure •Corticosteroids •Lymphoma (Hodgkin’s disease) •Aplastic anaemia |
|
|
Pllattelletts- characteristics
|
Pllattelletts (160-420,,000//mm3)
• Function – maintain integrity of blood vessels – interact to facilitate blood coagulation • provide specific receptor site for clotting factors • provide necessary phospholipid surface for conversion of prothrombin to thrombin • protection of thrombin from enzyme antithrombin |
|
|
Pllattelletts
• Production & metabolism |
Pllattelletts
• Production & metabolism – produced in bone marrow as megakaryocytes – megakaryocyte maturation & proliferation controlled by megakaryocyte colony stimulating factor (Mk-CSF) & thrombopoietin – 2/3 platelets in circulation, 1/3 in spleen – life of platelet 8 - 11 days – Spleen site of destruction |
|
|
Pllattelletts
• Decrease (Thrombocytopenia) |
Pllattelletts
• Decrease (Thrombocytopenia) – Decreased production – abnormal distribution – dilution – Causes include • neoplastic diseases • immune processes • infections • metabolic disorders • drugs & chemicals • Spleenomegaly • bone marrow failure eg Aplastic anaemia |
|
|
Pllattelletts
• Increases (thrombocytosis) |
Pllattelletts
• Increases (thrombocytosis) – part of reactive process – myeloproliferative disorder • Significant thrombocytosis may be associated with increased risk of vascular events |
|
|
Drug Induced Haemattollogiicall Diisorders - disgnosis, rechallenge, groups
|
Drug Induced Haemattollogiicall Diisorders..
• Uncommon event apart from cytotoxic drugs where it is the norm. • Diagnosis difficult as usually number of co-existing diseases, multiple drugs • Diagnosis often by exclusion • Rechallenge may not be possible/ethical • Broadly divided into 4 categories – Abnormal sensitivity to drug or metabolite – Genetic predisposition eg G6PD Deficiency – Abnormal metabolism with toxic metabolite – Immune mediated |
|
|
Aplastic Anaemia - requiremetns for classfication, what is it
|
Aplastic Anaemia
• Pancytopenia ie all cell lines affected • hypocellular marrow with no evidence of increased peripheral destruction • Requires at least 2 of WCC<3.5, Platelets<55, Hb < 10 with retics<30 • For drug induced, must be no infiltrates in bone marrow, no history of cytotoxic drugs or radiation exposure. |
|
|
Aplastic Anaemia - onset, incidence, cause
|
Aplastic Anaemia
Onset – usually slow – symptoms approx 6 weeks post exposure – Sx include fatigue, weakness, stomatitis, bruising, petechiae, purpura Incidence – approx 2.2 per 1,000,000 – higher with some drugs Aetiology – damage to haematopoietic stem cell – earlier stem cell is affected, the more severe and prolonged the aplasia |
|
|
Aplastic Anaemia
• Mechanisms |
Aplastic Anaemia
• Mechanisms – Dose related eg cytotoxics (predictable effect) – Idiosyncratic - most common – abnormal metabolism and excretion • eg Chloramphenicol - nitroso group from nitrobenzene ring reacts with stem cell DNA causing chromosome damage and cell death. • Phenytoin, Carbamazepine metabolites bind covalently to macromolecules causing stem cell or lymphocyte death • Phenylbutazone - ? Abnormal clearance Genetic Predisposition • ? Involved in Chloramphenicol aplasia – Immune reaction • eg Quinine affects on suppressor T cells could inhibit stem cell production suggested by responsiveness to antithymocyte globulin |
|
|
Aplastic Anaemia
Drugs more commonly involved include |
Aplastic Anaemia
Drugs more commonly involved include Acetazolamide OHA’s Antihistamines Phenylbutazone Carbamazepine Penicillamine Chloramphenicol Oxpentifylline Chloroquine Phenothiazines Thiazides Phenytoin Felbamate Propylthiouracil Frusemide Quinine/Quinidine Gold Salts Sulphonamides Indomethacin Ticlopidine Interferon alpha |
|
|
Aplastic Anaemia
Treatment |
Aplastic Anaemia
Treatment • Identify and remove cause, earlier the better • Symptomatic treatment eg antibiotics, blood products • Antithymocyte globulin (20mg/kg/d for 8 days) • Corticosteroids ? Efficiacy • Immunosuppressants - ? cyclosporin • Colony Stimulators - eg GM-CSF, G-CSF - some case reports of success with interleukin 1 • Bone Marrow transplantation |
|
|
Agranulocytosis- onset incidence
|
Agranulocytosis
Onset – usually rapidly – symptoms approx 7-14 days post initiation of therapy – Sx include sore throat, fever, fatigue, weakness,chills (resemble flu) stomatitis, Incidence – Varies; – More frequent in females – Mortality 16% overall; increased with organ failure and sepsis. |
|
|
Agranulocytosis
Mechanisms |
Agranulocytosis
Mechanisms – May be directly on mature granulocytes causing arrest of maturation or – toxic effect on myeloid colony forming units in bone marrow (? More likely) • 1. Immune mediated involving drug or metabolite, antibodies and neutrophils – Drug membrane complex acts as hapten to stimulate antibody formation eg high dose penicillin – Innocent bystander - drug/antibody complex absorbed onto neutrophil membrane causing complement activation and cell destruction eg Quinine – Complex of drug and protein carrier causing antibody activation; complex attaches to neutrophil surface causing cell destruction – Drug produces change in neutrophil surface which activates autoantibody formation 2. Accumulated drug toxicity in hypersensitive individual – Penicillins - high concs inhibit myeloid colony forming units – Antithyroid agents (PTU, Carbimazole) 0.3-0.6% patients, > in patients more than 40years – Phenothiazines - onset 2-15 weeks, total dose > 10-20g; most common females > 50years – Clozapine - 10 times more common than with Chlorpromazine; increases with age most common first 6 months; marker is steady decline in WCC; ? Free radicle metabolite • Type 111 reaction - combination immune & toxic mechanisms |
|
|
Agranulocytosis
Treatment |
Agranulocytosis
Treatment • Identify and remove offending agent • Symptomatic treatment and support while recovery • Severe cases may benefit from Immune Globulin 400mg/kg/d • Colony Stimulators (G-CSF, GM-CSF) may reduce time of neutropenia |
|
|
Agranulocytosis
Drugs involved include |
Agranulocytosis
Drugs involved include Acetazolamide Benzodiazepines Aspirin Allopurinol beta lactams ACEI’s Antithyroid agents Carbamazepine Chloramphenicol Cimetidine Clindamycin Clozapine Dapsone Doxycycline Hydralazine OHA’s Thiazides Frusemide Phenylbutazone Phenothiazines Penicillamine Quinine/Quinidine Indomethacin Sulphonamides Ganciclovir TCA’s Methyldopa Phenytoin Isoniazid Metronidazole Pyrimethamine Rifampicin Vancomycin HIV drugs etc etc |
|
|
process of agranulocytosis
|
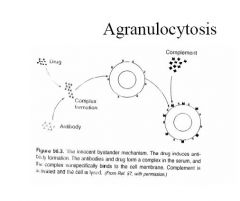
|
|
|
Drug iinduce Haemollytiic Anaemiia
|
Drug iinduce Haemollytiic Anaemiia
• Disorder where RBC’s damaged or destroyed • Destruction can occur – within blood vessel (intravascular haemolysis) • mainly Immune mediated – outside vascular space (extravascular haemolysis) • mainly metabolic mediated. • Anaemia occurs when destruction exceeds bone marrow production |
|
|
Drug iinduce Haemollytiic Anaemiia
Drugs implicated include |
Drug iinduce Haemollytiic Anaemiia
Drugs implicated include Cephalosporins & Penicillins Phenothiazines NSAID’s Thiazides Hydralazine Levodopa Melphalan Methyldopa Methysergide Omeprazole Probenecid Procainamide Quinine/Quinidine Rifampicin Sulphonamides Sulphonylureas Tetracyclines Triamterene etc |
|
|
Drug iinduce Haemollytiic Anaemiia
Immune Haemolytic Anaemia Mechanisms - similar to agranulocytosis |
1. Hapten Formation
2. Innocent bystander 3. Low Affinity Hapten 4. Protein binding |
|
|
Drug iinduce Haemollytiic Anaemiia
Immune Haemolytic Anaemia Mechanisms - similar to agranulocytosis hapten formation innnocent bystander |
Drug iinduce Haemollytiic Anaemiia
Immune Haemolytic Anaemia Mechanisms - similar to agranulocytosis 1. Hapten Formation – Drug absorbed onto RBC membrane to form hapten with antibody formation – RBC’s destroyed by phagocytosis or cell mediated cytotoxicity – seen with penicillins & cephalosporins, tetracyclines, cytotoxics (Cisplatin, Cyclophosphamide, Melphalan) 2. Innocent bystander – Drug forms complexes with drug specific antibodies in serum; – loosely adhere to RBC membranes; – activate complement system which lyses RBC membrane with release of drug/antibody complex back into serum where it may attach to other RBC’s – seen with Quinine, Quinidine |
|
|
Drug iinduce Haemollytiic Anaemiia
Immune Haemolytic Anaemia Mechanisms - similar to agranulocytosis 3. Low Affinity Hapten 4. protein binding |
Drug iinduce Haemollytiic Anaemiia
Immune Haemolytic Anaemia Mechanisms - similar to agranulocytosis 3. Low Affinity Hapten – drug or metabolite binds to antigenic sites on RBC membrane forming complete antigen or change to shape to unmask antigen site – activation of complement system leads to cell destruction and release of drug/metabolite for further damage. 4. Protein binding – drug combines with nonspecific protein eg Albumin, IgA, Fibrinogen – combination adheres to RBC – While this binding is not immunologic and usually does not cause haemolysis, the complex interferes with blood cross matching due to nonspecific binding of antibodies to RBC membranes eg Cephalosporins |
|
|
Drug iinduce Haemollytiic Anaemiia
Test for Haemolytic Anaemia |
1. Coombes Test
2. Haptoglobin 3. Haemopexin 3.Metabolic Haemolytic Anaemia G6PD deficiency- drug or genetically induced |
|
|
Drug iinduce Haemollytiic Anaemiia
Test for Haemolytic Anaemia 1. Coombes Test |
Drug iinduce Haemollytiic Anaemiia
Test for Haemolytic Anaemia 1. Coombes Test – Screening test to detect antibodies against RBC – Indirect Coombes - determining presence of antibodies in serum rather than attached to RBC – Direct Coombes - presence of Ab bound to RBC – eg Methyldopa - 25% patients have positive Coombes test, only 0.8% develop HA (? Methyldopa inhibits proliferation of nonspecific T cells thus allowing unregulated production of autoantibodies positive Coombes test. Methyldopa also may inhibit RE systems thus preventing HA ; patients without impairment of RE system may develop HA |
|
|
Drug iinduce Haemollytiic Anaemiia
2. Haptoglobin 3. Haemopexin |
Drug iinduce Haemollytiic Anaemiia
2. Haptoglobin • Circulating alpha globulin • Acts as carrier for haemoglobin to reduce toxicity of Hb • As large complex, not filtered by glomerulus • Delivers Hb to RE system for metabolism • Measure of haemolysis – No haemolysis - Normal – Moderate haemolysis - decreased – Severe haemolysis - markedly decreased or absent 3. Haemopexin • Beta globulin that binds free heme molecules • Measure of haemolysis – No haemolysis - Normal – Moderate haemolysis - normal or slightly decreased – Severe haemolysis - decreased or absent |
|
|
Drug iinduce Haemollytiic Anaemiia
3.2 Metabolic Haemolytic Anaemia |
Drug iinduce Haemollytiic Anaemiia
3.2 Metabolic Haemolytic Anaemia – Due to hereditary RBC defect with intravascular destruction – Oxidative haemolysis most commonly associated with Glucose-6-phosphate dehydrogenase (G6PD) deficiency – G6PD deficiency most common in Mediterranean population, less common in American blacks – Exposes patients to high risk of haemolysis |
|
|
Drug iinduce Haemollytiic Anaemiia
3.2 Metabolic Haemolytic Anaemia – drugs with high risk in G6PD deficiency include |
Drug iinduce Haemollytiic Anaemiia
3.2 Metabolic Haemolytic Anaemia – drugs with high risk in G6PD deficiency include Ascorbic Acid Aspirin Benzocaine Chloramphenicol Dapsone Diazoxide Methylene Blue Nitrofurantoin Phenopyridine Primoquine Sulphonamides – Careful evaluation of therapy essential in patients with G6PD Deficiency |
|
|
Drug induce Thrombocytopeniia
|
Drug induce Thrombocytopeniia
• Most common of drug induced haematological disorders. • Associated with a large number of drugs including Acetazolamide Aspirin Allopurinol Benzodiazepines Cephalosporins Cimetidine & H2 antagonists Digoxin Frusemide Heparins Hydroxychloroquine Interferon Isoniazid NSAID’s Morphine Phenothiazines Penicillins Thiazides Sulphonamides Phenytoin Procainamide Quinine/Quinidine Rifampicin Trimethoprim Sulphonylureas etc.etc |
|
|
Drug induce Thrombocytopeniia - when classifed
Mechanisms |
Drug induce Thrombocytopeniia
• Thrombocytopenia defined as platelet count < 100,000/mm3 • Complications unlikely until < 30,000 • Symptoms vary with severity - early signs bruising, petechiae, epistaxis, with frank bleeding as worsens Mechanisms • Similar to those seen with other haematological disorders – immune mediated • increased peripheral destruction of platelets; increased megakaryocytes in bone marrow – direct toxicity • effects in the bone marrow; decreased megakaryocyte production |
|
|
Drug induce Thrombocytopeniia
Specific examples 1. Heparin |
Drug induce Thrombocytopeniia
Specific examples 1. Heparin – Occurs in up to 25% patients; any dose or route – 2 distinct types • Mild form occurs early, with platelet count < 100,000; no symptoms; resolves rapidly on cessation (? Due sequestration of platelets) • Severe form; likely immune mediated with antibody formation to platelet/heparin complex leading to Platelet destruction – usually occurs late (6-12 days) with – platelets fall to < 30,000, – associated with bleeding, & worsening of thrombosis. Platelet antibodies attach to endothelial cells on the walls of blood vessels leading to thrombosis – Rate reported at 1-1.3% with porcine Heparin. – Less common with LMWH’s but still possible; Danaparoid probably not implicated. |
|
|
Drug induce Thrombocytopeniia
Specific examples 2. Gold Salts 3. Quinine/Quinidine |
Drug induce Thrombocytopeniia
Specific examples 2. Gold Salts – antibody formation to platelets – Incidence 1-3% – Often abrupt and severe, but may be delayed and even occur months after cessation of Gold 3. Quinine/Quinidine – Various mechanisms including Innocent bystander, hapten reaction, antibodies against platelet membrane glycoproteins – May occur 7-10days after first exposure, but subsequent treatment more likely to precipitate full blown event – Minute doses may precipitate thrombocytopenia (eg bitters) – Rapid fall in platelets with early bleeding – Signs include petechiae, purpura, oral haemorrhagic bullae, overt bleeding |
|
|
Drug induce Thrombocytopeniia
Specific examples 4. Thiazides 5. alcohol |
Drug induce Thrombocytopeniia
Specific examples 4. Thiazides – direct bone marrow suppression usually, although antibodies have been identified. 5. Alcohol – acts as general bone marrow suppressant – chronic alcohol intake decreases production, decreases platelet survival, and platelet dysfunction – Rates - hospitalised alcoholics 26%, well patients 3% |

