![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
modern protein separation methods rely heavily on... |
chromatography. |
|
|
state how chromatography works... |
the mixture of substances to be separated is dissolved in a liquid or gaseous fluid (mobile phase) percolated through a column consisting of a porous solid matrix (secondary phase). individual substances are retarded at different rates, resulting in the mixture to separate into bands of pure substances. |
|
|
chromatography can be classified accordingly to their mobile and stationary phase... |
most techniques described below will be solid-liquid chromatography. |
|
|
chromatography is further classified by the nature of the dominant interaction between the substances and the stationary phase. List the 4 types of chromatography... |
1) ion exchange chromatography 2) hydrophobic chromatography 3) gel-filtration chromatography 4) affinity chromatography |
|
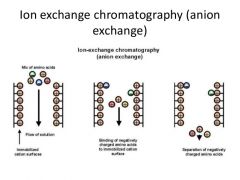
ion-exchange chromatography... |
In ion exchange, ions that are electrostaticaly bound to an insoluble and chemically inert matrix are reversibly replaced by ions in solution. Different proteins have different affinity for the ion exchanger, (matrix) depending on their net charges. Separation on the basis of ionic charge |
|
|
the affinity of a protein (polyelectrolyte) to a ion exchanger depends on: |
1) identity and concentrations of other ions in solution because of the competition amongst various ions. 2) pH because the net charges of acid-base groups vary with pH (At a pH below their isoelectric point proteins carry a net positive charge, at a pH above, proteins carry a net negative charge) |
|
|
in ion exchange you can decrease the affinity of the ions for the matrix by... |
increasing the salt concentration in the buffer. |
|
|
the second type of chromatography is gel-filtration chromatography which separates mixtures on the basis of... |
size and shape. |
|
|
The molecules which are too big to enter the pores of the gel beads of the column are eluted in a smaller elutant volume than the smaller molecules that pass through the pores... |
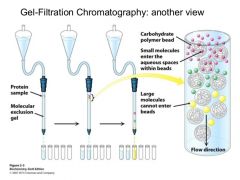
thus small molecules are retarded- their journey is made longer compared to larger molecules which leave the column first---> separation |
|
|
some definitions relating to gel filtration/ size exclusion chromatography... |
exclusion limit - the smallest molecule unable to penetrate the pores of a given gel is said to be the gel's exclusion limit. |
|
|
the elution volume of a given solute (protein) Ve is... |
the volume of the solvent required to elute the solute from the column. e.g a new protein elutes the column after 14mls of buffer is added, thus Ve = 14mls |
|
|
the void volume (Vo)... |
is the volume of the solvent space surrounding the beads, and can be easily measured. |
|
|
the relative elution volume, Ve/Vo... |
characterises each solute and can be used to estimate molecular masses. |
|
|
the third type of chromatography is affinity chromatography which separates proteins... |
on the ability of protein to bind to particular small molecules or other macromolecules. |
|
|
many proteins can bind specifically to other molecules, in a very high affinity, but not covalenty. This property is exploited in affinity chromatography... |
A ligand, that binds specifically to a protein of interest is attached covalenty to a resin: when an impure solution is passed through, the desired protein binds but all the others are washed away. The desired protein is then released changing elution conditions. |
|
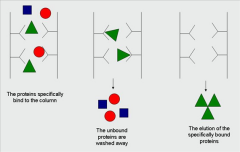
|
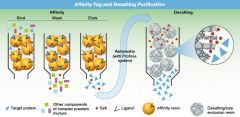
there are different types of affinity chromatography: 1) immunoaffinity chromatography 2) Glutathione-S-transferase; glutathione 3) insulin; insulin receptor 4) glucose; glucose binding protein 5) Metal chelation: His-tag and divalent metal ions (Ni2+, Co2+, Zn2+) |
|
|
A fourth type of chromatography is hydrophobic chromatography (HIC; hydrophobic interaction chromatography)... |
HIC separates native proteins on the basis of surface hydrophobicity. the stationary phase is a hydrophilic substance lightly substituted with hydrophobic groups (octyl of phenyl residues)... the result are weak interactions that maintain the native fold of the protein. |
|
|
Elution is achieved by weakening progressively the hydrophobic interactions, for example... |
aqueous solutions with decreasing salt concentrations. *increasing salt concentration = stronger hydrophobic interactions salt=hydrophobic |
|
|
reverse phase chromatography uses... |
liquid-liquid chromatography. used to separate mixture of non-polar substances. |
|
|
in reverse phase chromatography the stationary phase consists of... |
a liquid immobilised on a silica substituted with C8 and C18 alkyl chains and the mobile phase is a more polar liquid |
|
|
reverse phase is used to... |
separate mixture of non-polar substances. For proteins, denaturation results in exposure of hydrophobic side chain. |
|
|
the elution phase should be highly... |
non-polar (e.g high concentrations of acetonitrile) to dislodge the substances from stationary phase. |
|
|
High performance liquid chromatography (HPLC) has greatly improved separations... |
-high resolution -high speed -high sensitivity -automation |
|
|
analysis of the fraction eluted from chromatography columns is carried out by... |
gel electrophoresis. (verify how many proteins we actually have) electrophoresis is the migration of ions in an electric field. It is widely used for separation of biological macromolecules (proteins) |
|
|
F=vf where v is the rate of migration (velocity) of the ion and f is the frictional coeffcient. F depends on size, shape and the size of solvation of the ion as well as from the viscosity of solution. |
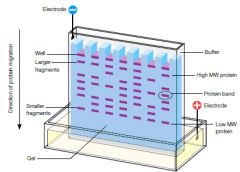
small proteins migrate faster large proteins migrate slower |
|
|
Gel electrophoresis is one of the most powerful and used methods for macromolecular separation... |
the gels in common use are polyacrylamide and agarose. They have pores of molecular dimension whose size can be specified/ In polyarcylamide gel electrophoresis (PAGE) gels are made by the free radical polymerisation of acrylamide and N,N- methylenebisacrylamide. |
|
|
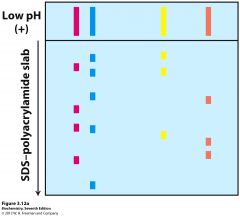
SDS-PAGE electrophoresis (sodium dodecyl sulfate, polyarcylamide gel electrophoresis)... |
SDS is a powerful negatively charged detergent, that binds to proteins and unfolds them. SDS masks the protein's intrinsic charge and causes it to migrate towards the positive electrode. Proteins are separated on the basis of their molecular masses and are visualised with a dye. |
|
|
what is the name of the reducing agent, that is normally added to break any S-S linkages in proteins? |
B-mercaptoethanol. |
|
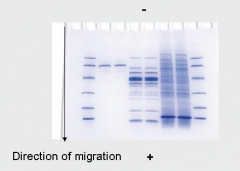
proteins are separated on the basis of their molecular masses... |
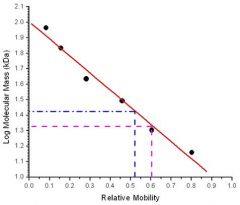
the relative mobility of proteins in such gels vary linearly with the logarithm of their molecular masses (larger the log of molecular mass, the reduced mobility) SDS PAGE electrophoresis can determine molecular mass to an accuracy of 5-10% |
|
|
proteins can also be separated electrophoretically on the basis of their native content of acidic and basic residues (ionic content). This is known as... |
isoelectric focusing. |
|
|
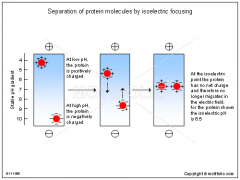
define isoelectric point... |
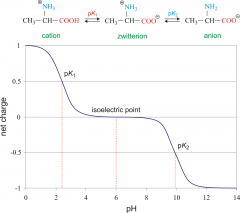
of a protein is the pH at which its net charge is zero. At this pH the electrophoretic mobility is zero. |
|
|
In isoelectric focusing, the pH gradient in the gel is first formed by... |
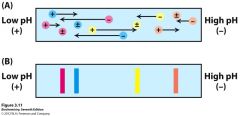
subjecting a mixture of polyampholytes (small mulitcharged polymers) having many different isoelectric values to electrophoresis. |
|
|
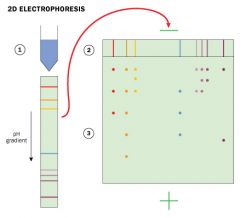
Isoelectric focusing can be combined with SDS PAGE electrophoresis to obtain high resolution separations - Two dimensional gel electrophoresis. |
|
|
A sample is subjected to isoelectric focusing: separation on basis of proteins' intrinsic charges... |
the single lane gel is then placed on the top of the polyacrylamide gel and run in a direction that is at a right angel to previous one. SDS is added and proteins separate according to their size. The only proteins that remain unresolved are the ones with identical isoelectric points and size, a relatively rare situation. |
|
|
|
|
|
To summarise, proteins are fractionated (separated) by column chromatography, proteins are retarded to different extents by their interaction with the matrix. Proteins can be separated according to... |
1) their charge (ion-exchange chromatography)- the strength of association between the dissolved molecules and the ion-exchange matrix depends on the ionic strength and pH of the solution that is passing through the column. 2) their hydrophobicity (hydrophobic chromatography) 3) their size (gel-filtration chromatography) 4) their ability to bind to particular small molecules (affinity chromatography)- insoluble matrix is an antibody molecule or an enzyme substrate that will bind to a specific protein. |
|
|
the most efficient chromatography method is... |
affinity chromatography, which takes advantage of the biologically important binding interactions that occur on protein surfaces. High degrees of purification can be achieved in a single pass through a affinity column. |
|
|
In typical purification, the sample is passed through several different columns in turn- the enriched fractions obtained from one column are applied to the next... |
recombinant DNA techniques, allow special recongnition tags to be attached to proteins, thereby greatly simplifying purification. |
|
|
After chromatography, further separation can be carried out by SDS PAGE electrophoresis... |
SDS PAGE uses a highly-cross linked gel of polyacrylamide as the inert matrix through which the proteins can migrate. SDS (sodium dodecyl sulfate) is a powerful negatively charged detergent---> binds to hydrophobic regions of the protein molecules causing them to unfold into extended polypeptide chains. In electrophoresis, large proteins retard more than small ones. SDS PAGE is widely used because it can separate all types of proteins, including those that are normally insoluble in water- such as many proteins in the membrane. an electric field is applied to the gel so that proteins migrate form the negative to the positive electrodes, typically from top to bottom. |
|
|
In 2D electrophoresis separation... |
1) proteins are first separated on the basis of their isoelectric points by isoelectric focusing from left to right- at isoelectric point, the protein has no net charge and therefore does not migrate in an electric field, each protein moves to a position in the gradient that corresponds to its isoelectric point and remains there. 2) Then further fractionated according to their molecular weights by electrophoresis in the presense of SDS |

