![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What Is the Partical Theory Of Matter? |
Spead , unique, movement, attract, size |
SUMAS |
|
|
What is matter |
Substance that has mass and volume |
|
|
|
What is a Physical Property |
Any property that can be measured |
|
|
|
What is a Chemical Property |
A characteristic that becomes evadent after or during a chemical reaction |
|
|
|
Quantitative property |
A characteristic or a substance that can be measured |
|
|
|
Qualitative Property |
A characteristic of a substance that can be discribed not measured |
|
|
|
Density |
The amount of Mas in a certain unit of mass |
|
|
|
Element |
Pure substance that can not be broken into simple parts by ordanary chemical means |
|
|
|
Compound |
A pure substance made up of 2 or more substances |
|
|
|
Atomic number |
Number of protons in the nucleus of an atom |
|
|
|
Atomic mass |
Unit used to measure mass of an atom |
|
|
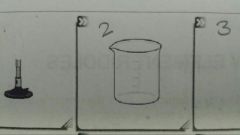
What is this |
A beaker |
|
|
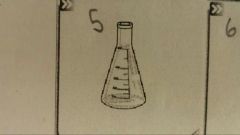
What is this? |
An Erlenmeyer Flask |
|
|
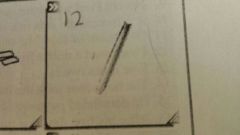
what is this |
A scoopula |
|
|
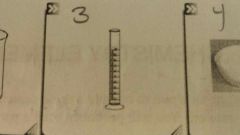
What is this? |
A graduate cylinder |
|
|
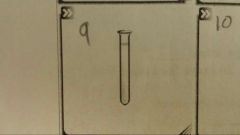
What is this? |
A test tube |
|
|

What symbol is this |
Compressed gas |
|
|

What symbol is this? |
Flammable and Combustible |
|
|

What symbol is this? |
Oxidizing Material |
|
|
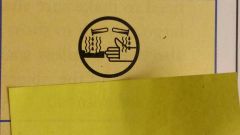
What symbol is this? |
Corrosive |
|
|

What symbol is this |
positions and infections, Immediat and serious |
|
|

What symbol is this |
Poisonous and infectious causing other toxic effects |
Simaler to another but look at the T |
|

What Is this symbol? |
Biohazordous and infectious |
|
|

What Is this symbol? |
Dangerously Reactive |
|
|
|
Where is the proton located |
In the nucleus |
|
|
|
Where are neutrons |
In the nucleus |
|
|
|
Where are electrons |
On the outer rings of an atom |
|
|
|
Where are electrons |
On the outer rings of an atom |
|
|
|
Charge of an proton |
Positive |
|
|
|
Charge of an neutron |
Neutral |
|
|
|
Charge of an electron |
Negative |
|
|
|
What are the names of the rows and columns on the periodic table |
Rows are called periods and the columns are called groups |
|
|
|
What is the forumula for density |
Mass ÷ volume |
|
|
|
What are 3 properties of metals |
Shinie, Ductile, malubule, conduct electricity |
|
|
|
What are 3 properties of non-metals |
Brittle, non ductile, non malubule not very shinnie |
|
|
|
3 properties of metalloids |
conduct heat and electricity better then non metals but worse then metal , Ductile,malubule, can be shinnie of dull |
|
|
|
Where are alkali metals located |
Very left on group (row) 1 |
|
|
|
Where r are alkaline earth metals located |
Left row 2 |
|
|
|
Where are noble gasses located |
Very right row 18 |
|
|
|
Where are halogens |
Right row 17 |
|
|
|
How reactive are alkali metals |
Verry |
|
|
|
How reactive are alkaline earth metals |
Not as reactive as alkali |
|
|
|
How reactive are noble gasses |
Stable |
|
|
|
outer electrons of alkali metals |
1 |
|

