![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Naturally occurring silver is 51.84% silver-107 and 48.16% silver-109. Calculate the relative atomic mass of silver. |
r.a.m. (Ag) = (51.84/100 x 107) + (48.16/100 x 109) = 55.469 + 52.494 = 107.96 |
(x%/100xAM) + (x%/100xAM) =r.a.m |
|
|
Copper consists of two isotopes, copper-63 and copper-65. Its relative atomic mass is 63.62. Find the abundance of each isotope. |
Let y/100 = the abundance of copper-63 63.62 = (y/100 x 63) + [(100 - y)/100 x 65] Abundance of copper-63 = 69/100 = 69% Abundance of copper-65 = 100 - 69 = 31% |
|
|
|
Define/Draw: Element, Molecule, Proton, Neutron, Electron, Mass number, Atomic Number, and Chemical Symbol. |
uncomplete
|
|
|
|
How is an atom/element defined? |
By its atomic number, aka # of protons |
|
|
|
What is an Isotope? |
Atoms w/ same atomic number but different numbers of neutrons in nuclei |
See Isotopes of Hydrogen |
|
|
59
26 FE List the electrons, neutrons, and protons. |
uncomplete |
Atomic number (Z)= no. of p Mass number (A) = no. of p + no. of n
For neutral atom, no. of e− = no. of p |
|
|
What do Ions give? Define a Cation and an Anion with an example |
Ions are specific chemical species that have either a positive or negative electric charge • Cations have positive (+) charge • Anions are negatively (−) charged
|
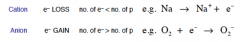
|
|
|
How many grams are 103 mg? |
103mg x 1/1000mg = 0.103g |
Prefix m = milli = x 10 -3 |
|
|
How many metres are in 1 nm? |
1nm x 1x10-9m/1nm = 1x10-9m |
Prefix n = nano = x 10-9 |
|
|
Conv Celcius to Kelvin |
30+270 = ___Kelvin |
oC + 273 |
|
|
Define: precision vs accuracy |
Accuracy: how close the value is to the correct or accepted value
|
|
|
|
• 15 = 1.5 x 101 • 362 = 3.62 x 102 • 0.0023 = 2.3 x 10-3 • 0.00230 = 2.30 x 10-3 • 0.00203 = 2.03 x 10-3
• 2.3 mm = 2.3 x 106 nm |
2 sig. figs. 3 sig. figs. 2 sig. figs. 3 sig. figs. 2 sig. figs. Sig. figs. are the same regardless of units (as precision in measurement does not depend on the units in which it is expressed)
3 sig. figs. |
|
|
|
a. 1.000405 b. 0.001000 c. 1000010.0 |
a) 1.000405 x 10 0 b) 1.000 x 10−3 c) 1.0000100 x 10 6 b) 4 sig. figs. c) 8 sig. figs. |
|
|
|
Define: Hydrated compunds with the different types |
Anhydrous and Hydrates |
|
|
|
The moles abbreviation, other names, and SI Unit |
Abbreviation: mol, Avogadros Number(NA) or Avogadros constant. SI Unit = 6.022x10 23 |
|
|
|
One mole of C-12 weighs how much in grams? |
12g (RAM) |
|
|
|
6.022 x 10 23 atoms of natural silicon has the weight of what in g? |
28.08g (RAM) |
|
|
|
Define the term: Molar mass and include the formula for finding the Molar Mass? |
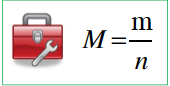
Molar mass is defined as the mass of 1 mole of the specified substance (units g mol─1, i.e. grams per mol) – Molar mass of an atom is often referred to as its atomic mass (available from periodic table) – Molar mass of a compound or molecule equals the sum of the
atomic masses of all the atoms present in the substance |
M=Molar Mass m=Mass in G n=# of moles |
|
|
How many moles of molecular sulfur (S8) are present in
35.6 g? The atomic mass of sulfur, S is 32.06 g mol-1. |
If: M=m/n then, n x M=m If: n x M = m then n= m/M |
If: M=m/n then, n x M=m If: n x M = m then n= m/M |
|
|
Calculate the % natural abundance of the isotope 22Ne if a 12.4 g sample of neon gas is found to contain
3.30 x 1022 atoms of 22Ne. |
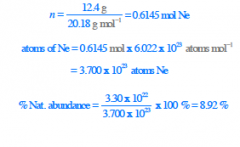
|
|
|
|
Define Molar Ratio: Give an Example |
For chemical compounds, mole ratios are the same as
the ratios of the individual atoms, e.g. for P4O10 |
|
|
|
What mass of iron is required to react with 25.6 g of O to make Fe2O3, assuming that the two reactants are used completely to form
Fe2O3? |
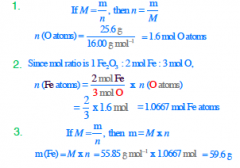
|
|
|
|
Formula Triangle 1) |
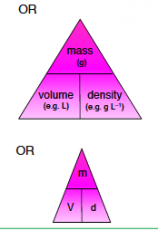
|
|
|
|
Formula triangle 2) |
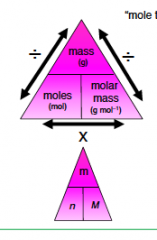
|
|
|
|
Formula triangle 3) |
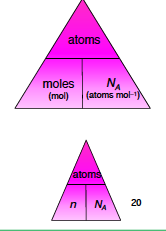
|
|
|
|
Other Chemical conversions |

|
|

