![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
Matter |
-Anything that has mass and occupies space -The particle theory of matter states that all matter is made up of tiny particles - Matter is divided into two categories pure substances and mixtures - Matter can be found many states from solids, liquids, and gases |
|
|
Pure Substance |
- This is matter that contains only one kind of particle and can't be broken down further by physical methods. - They are further categorized into elements and compounds - Pure substances, especially compounds, are chemically bonded - They can include tin (Sn), water (H2O) , table salt (NaCl), nitrogen(N), or baking soda (NaHCO3). |
|
|
Mixture |
-Matter that contains more than one kind of particle - They include Homogenous (Solution) and Heterogeneous (Mechanical) mixtures - The particles in a mixture are physically bonded so they can be separated by physical methods - These physical methods include filtering, distillation or even magnetism
|
|
|
Element |
- A pure substance that cannot be broken down into simpler parts by chemical or physical methods - The only way an element can be seperated by a particle collider - Each element has its own unique number of protons than other elements that have their own unique number of protons |
|
|
Compound |
-A pure substance made of two or more different elements that are chemically combined, and can be broken down by chemical methods. - They can include water (H2O) or baking soda(NaHCO3) |
|
|
Physical Property |
-A characteristic of a substance that can be observed and measured without changing the identity of the substance. -Physical property are divided into qualitative and quantitative physical properties. -Qualitative physical properties can be observed and described without any detailed measurements. They include colour,odour,state,texture,lustre,and malleability. -Quantitative physical properties can be measured and can be assigned a particular value. They include viscosity, melting point, boiling point,solubility,hardness,conductivity,and density |
|
|
Viscosity |
The measure of a substance's resistance to flow |
|
|
Melting point |
The temperature at which a solid turns into a liquid |
|
|
Boiling point |
The temperature at which a liquid turns into a gas |
|
|
Solubility |
A measure of the ability of a substance to dissolve in another substance |
|
|
Density |
The ratio of the mass of a substance to the volume it occupies |
|
|
Chemical Property |
Describes the behaviour of a substance as it reacts with another substance to form one or more new substances |
|
|
Combustibility |
The chemical property of a substance to react with oxygen to produce carbon dioxide or it is the ability of a substance to burn in the air |
|
|
Stability |
The ability of a substance to remain unchanged |
|
|
Toxicity |
The ability of a substance to cause harmful effects in plants and animals |
|
|
Atom |
-The smallest particle of an element that retains the identity of the element -All matter is made up of billions of tiny atoms -Atoms are made of subatomic particles that include electrons, protons, and neutrons |
|
|
Subatomic particle |
A particle that is smaller than the atom they include electrons, protons, and neutrons |
|
|
Electron |
-A negatively charged particle within the atom - It has a relative mass of 1 -Revolves around the nucleus in energy levels -An electron can only move up an energy level by gaining an energy called quantum |
|
|
Nucleus |
In chemistry, the positively charged center of an atom that is a lump of protons and neutrons that are bonded together and the only way to separate a nucleus is by using a particle collider. |
|
|
Proton |
-A positively charged particle that is part of every atomic nucleus - Has a relative mass of 1836 -The number of protons in an atom determines what element is that atom |
|
|
Neutron |
-An uncharged particle that is part of almost every atomic nucleus -Has a relative mass of 1837 - The number of neutrons makes a difference in an element's mass number together with protons - Also, the number of neutrons affects whether or not an atom is an isotope of a specific atomic mass that is different from the atom that the number of neutrons has increased or decreased |
|
|
Energy Levels |
A possible level of energy an electron can have in an atom and the only way an electron can move up an energy level is gathering enough energy called quantum. The concept of energy levels around the nucleus was first proposed by a scientist called Niels Bohr |
|
|
Atomic number |

The number of protons in the nucleus of an atom that determines what element does the atom belong to. It can be found on the top left corner of an element's box.
|
|
|
Mass number |
The sum of the number of protons and the number of neutrons in the nucleus of an atom |
|
|
Isotope |
One of two or more forms of an element that have the same number of protons but a different number of neutrons |
|
|
Bohr-Rutherford Diagram |
A model of the atom in which a central positive nucleus is surrounded by electrons in energy levels |
|
|
Ion |
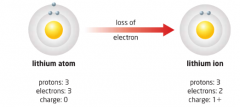
When an atom loses or gains electrons, the balance between positive and negative charges no longer exists and the atom becomes charged and this is called an ion |
|
|
Atomic Mass |

The average mass of the naturally occurring isotopes of an element this can be found on the bottom of an individual element's box |
|
|
Periodic Table |
A system for orgainzing the elements into columns and rows, so that elements with similar properties are in the same column |
|
|
Metal |
Typically, an element that is hard, shiny, malleable, and ductile, and is a good conductor of heat and electricity. These can be mostly found on the left of the periodic table. These include tin, aluminium, or zinc |
|
|
Non-metal |
Typically, an element that is not shiny, malleable, or ductile, and is a poor conductor of heat and electricity. These can be found onthe right of a periodic table. They include Helium, Nitrogen or Iodine. |
|
|
Metalloids |
An element that shares some properties with metals and some properties with non-metals. They include Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, and Astaine. |
|
|
Period |
A horizontal row of elements on the periodic table. These are numbered from 1 to 7, beginning in the first row of the periodic table and moving downward. |
|
|
Group |
A vertical column of elements in the periodic table that are numbered from 1 to 18 begins at the left-hand side of the periodic table across the right-hand side. - A group is also called a family because the elements in a group tend to have very similar physical and chemical properties. |
|
|
Alkali Metal |
These are metals are the elements in Group 1 (except for hydrogen). These metals ahve low melting points. They are soft enough to be cut with a knife. They are highly reactive so these metals are stored in kerosene or oil to keep water and oxygen away. |
|
|
Alkaline Earth Metal |
These metals are elements found in Group 2 . These metals are highly reactive, but they are less reactive than alkali metals. When they are heated, they will burn in the air to produce bright and colouful flames |
|
|
Halogens |
These non-metals are elements in Group 17 that are highly reactive and highly corrosive. As you move down Group 17, the melting points of the elements increases. They are normally solid at room temperatures. |
|
|
Noble Gases |
These non-metals in Group 18 are all odourless, colorless gases at room temperatures. The main property of noble gases is their non-reactivity. They include Helium, Neon, Argon, Krypton, Xenon, and Radon |
|
|
Valence Electron |
An electron in the outermost occupied energy level of an atom. - Noble gases have a full set of valence electrons. For example, helium atoms have a full set of two valence electrons and the other noble gases have a full set of 8 valence electrons. -During a chemical reaction an atom can join with another atom by gaining, losing, or sharing valence electrons. |
|
|
Chemical Bond |
A chemical link between two atoms, which holds the atoms together. - One type of chemical bond is the ionic bond, which is formed by the attraction between oppositely charged ions. |
|
|
Ionic Bond |
A chemical bond that forms between oppositely charged ions - Ionic bonds form when one atom gives up one or more electrons to another atom |
|
|
Ionic Compound |
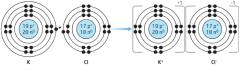
A compound made of oppositely charged ions |
|
|
Molecular Compound |
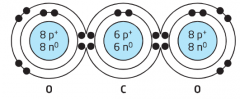
A compound formed when atoms of two or more different elements share electrons to create stability with a full outer energy level of electrons |
|
|
Covalent Bond |
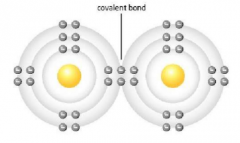
A chemical bond in which one or more pairs of electrons are shared by two atoms In a covalent bond, the shared electrons are attracted to the nuclei of both atoms. So the attraction of the nuclei for the shared electrons holds both atoms together. Unlike ionic compounds, the atoms remain uncharged |
|
|
Molecule |
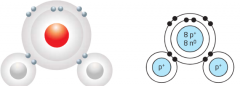
The smallest discrete particle of a pure substance, which has one or more shared pairs of electrons |

