![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
Evidence for Kekule’s model to be wrong: |
All C-C bond lengths are the same length, between C-C and C=C.
Only reacts with Br2 with a halogen carrier Benzene is lower in energy than Kekule’s structure suggests its should be. |
|
|
Discuss the structure and bonding in benzene / (comparing to kekule - structure):
|
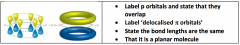
|
|
|
Discuss the relative low reactivity of benzene / (problems with Kekule – reactivity):
|

|
|
|
Discuss the reactivity of benzene compared to alkenes
|
|
|
|
Discuss the reactivity of phenol compared to benzene
|
|
|
|
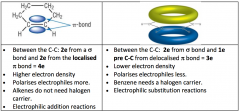
Benzene VS. Cyclohexene
|
Cyclohexene
Electrophillic Addition Electrons are localised Between C-C 2e from bond and 2e from localised bond = 4e Higher electron density, polarises electrophiles more Don’t need a halogen carrier Benzene Electrophillic Substitution Electrons are delocalised Between C-C 2e from bond and 1 e from the C-C from delocalised bond = 3e Lower electron density, polarises electrons less Need a halogen carrier |
|
|
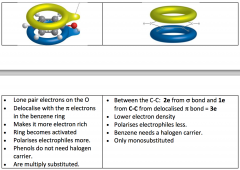
Benzene VS. Phenol
|
Phenol- Multiple Substitution
Lone pair of electrons on O Delocalise with the electrons in the Benzene ring Makes more electron rich Ring becomes activated, polarises electrophiles more Phenols do not need a halogen carrier Benzene- Mono-substitution Electrophillic Substitution Electrons are delocalised Between C-C 2e from bond and 1 e from pre C-C from delocalised bond = 3e Lower electron density, polarises electrons less Need a halogen carrier |
|
|
Nitration of benzene:
|
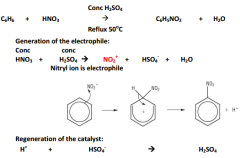
|
|
|
Halogenation of benzene:
|
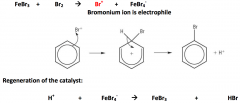
|
|
|
Carbonyl Test
Test for Carbonyl Group |
2,4,DNPH (Brady’s Reagent)
If present, orange precipitate formed FILTER, RECRYSTALISE, FILTER, MELTING POINT DETERMINATIO0N / COMPARE TO KNOW DATA |
|
|
Test to distinguish between Aldehyde and Ketone
|
Warm with Tollens Reagent (silver nitrate dissolved in ammonia)
If aldehyde present, silver mirror forms as the aldehyde is oxidised If ketone present no change as ketone cannot be oxidised |
|
|
Reduction of Aldehydes / ketonesMechanism of reducing an Aldehyde
|
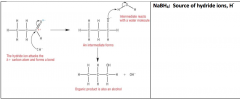
|
|
|
Azo Dyes
|
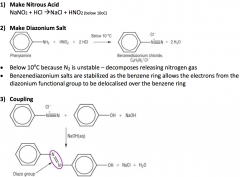
The Azo dye is now stable as there is extensive delocalisation over both arenas via the azo group, -N=N-
This also gives rise to the colours |
|
|
Amines
|
A weak base because of lone pair of electrons on N accept protons
proton acceptors lone pair electrons are donated forming a dative covalent bond |
|
|
Inductive Effect
|
Alkyl groups- positive inductive effect – stronger base
The alkyl group gives a small push of electrons towards LP on the N This makes it form a dative covalent bond more readily Ammonia - no inductive effect as nothing attached to functional group Benzene Ring – Negative inductive effect Benzene ring has small pull of electrons away from Nitrogen atom The LP electrons are delocalised into the benzene ring Makes them less readily available to form a dative covalent bond Weaker base |
|
|
Fatty acid - shorthand:
|
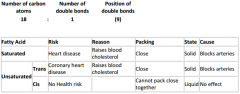
|
|
|
Trans fats cholesterol:
High Density Lipoproteins |
Carry cholesterol out of the blood and out of the body
Good |
|
|
Low Density Lipoproteins
|
Carry about 65% of cholesterol around the body and deposit lipids onto artery walls
This restricts blood flow Bad |
|
|
Making Biodiesel - Transesterification:
|
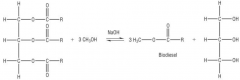
The waste oil is filtered then reacted with methanol and sodium hydroxide (catalyst) to form
biodiesel. This also increases the atom economy of fats. |
|
|
Preparation of Alphatic Amines
|
Warm halogenoalkens with excess ammonia
CH3CH2Cl + NH3 >>> CH3CH2NH2 + HCl NH3 + HCl >>> NH4Cl |
|
|
Preparation of Primary/Secondary aliphatic amines
|
CH3CH2NH2 + CH3CH2Cl >>> (CH3CH2)2NH
(CH3CH2)2NH + CH3CH2Cl >>> (CH3CH2)3N |
|
|
Isoelectric Point
|
Usually PH6 as COOH is slightly more acidic that NH2 is basic
Depends on side groups, hence the different points |
|
|
Acid Hydrolysis
|
Heat under reflux with 6mHCl for 24 hours
Always gives COOH and NH3+ |
|
|
Alkali Hydrolysis
|
Solution of NaOH, reflux
Always gives COO-Na+ and NH2 |
|
|
Hydrolysis of Polyesters/Polyamides
|
Hot aq Acid/ aq Alkali
As above for acid / alkali hydrolysis products |
|
|
Photodegradable polymers
|
Blended with light sensitive catalysts so become weak, brittle when exposed to light
Can also have C=O which absorb UV light and break Photodegradable plastics break to form shorter waxy hydrocarbon molecules before bacteria breaks them further into CO2 and H2O |
|
|
Chromatography
|
Chromatography
|
|
|
Stationary phase
|
is in a fixed place (paper in paper chromatography)
molecules interact with stationary phase slowing down their movement – ADSORPTION |
|
|
Mobile phase
|
moved in a definite direction (water rises up in paper chromatography)
molecules interact with mobile phase speeding up their movement – SOLUBILITY |
|
|
Thin Layer Chromatography – TLC
|
Dissolve sample.
Draw a pencil line and spot sample using a capillary tube, allow to dry. Place plate in a tank of solvent - solvent must be below line, seal the tank. Separation is by adsorption - allow solvent to almost reach the top, draw a line here - solvent front. |
|
|
Limitations of TLC
|
Similar compounds often have too similar Rfvalues.
Unknown compounds have no Rf value for comparison. It is hard to find a solvent that will have the correct amount of solubility - Goldilocks!! |
|
|
Rf Values
|

|
|
|
Gas Chromatography - GC
|
Gas Chromatography - GC
Is used to separate volatile compounds (gases) in a mixture with low boiling points |
|
|
The stationary phase:
GC |
Depends what is separated whether you use a liquid or solid lining of the chromatography column
e.g liquid long chain alkane (high boiling point) e.g solid silicone polymer |
|
|
The mobile phase:
GC |
Inert carrier gas e.g helium or nitrogen.
|
|
|
Separation
GC |
Different components slowed by different amounts- separation – retention times
Each component leaves the column at a different time and is detected as it leaves the column. Each peak represents a component Area under each peak is proportional to the abundance of each component |
|
|
Limitations of gas chromatography:
|
Similar retention times + peak shapes most compounds cannot be positively identified.
Not all substances can be separated. Unknown compounds have no reference retention times. Due to the limitations, gas chromatography is usually used in conjunction with spectroscopy. |
|
|
Uses for GC-MS
|
1) Forensics - scenes of crime
2) Environmental analysis - air pollutants, waste water, pesticides in food. 3) Airport security - explosives in luggage / airport security 4) Space probes - planetary atmospheres |
|
|
Chiral Compound
|
Optical isomers are one type of Stereo isomers (cis / trans is the other).
They are non-superimposable Chiral carbon has 4 different groups attached Always draw in 3D |
|
|
Properties of optical isomers:
|
They rotate plane polarised light.
One isomer rotates it in one direction and the other in the opposite direction |
|
|
Problems with Chiral drugs
|
One optical isomer may have serious side effects
Expensive/ difficult to separate isomers One optical isomer may have serious side effects Reduces the effectiveness Dose size |
|
|
Overcoming Chiral drug synthesis problems
|
Use and enzyme catalyst
Chiral Synthesis Chiral Catalyst Chiral Pool Synthesis |
|
|
1) Using enzymes as biological catalysts:
|
Nature is steroespecific, if this can be used only one isomer will be produced.
If a biocatalyst is used it will only catalyse the production of one isomer. |
|
|
2) Chiral pool synthesis:
|
This starts the synthesis pathway with a stereospecific enantiomer
All of the following synthesis steps should lead to a pure optical isomeric drug |
|
|
3) Use of transition metal complexes:
|
Some act as catalysts that will produce only one optical isomer
|
|
|
Cause of Stereo Isomerism
a) Optical isomerism |
C with 4 different groups attatched
Mirror images of each other |
|
|
b) Cis trans isomerism
|
C=C has restricted rotation
Both C in C=C attached to two different groups |
|
|
NMR:
Interpretation: always gives you 2 pieces of information |
1) splitting pattern – number of adjacent H’s
2) Chemical shift – adjacent functional groups If the numbers of H’s are given above the peak, make sure you use these too |
|
|
D2O
|
D replaces H in OH and NH protons
Peak for OH / NH protons disappears This is due to D having 2 nucleons – no signal |
|
|
TMS
|
Reference Signal at 0
|
|
|
Draw the structure of an optically active carboxylic acid with molecular formular of (C6H12O2). with five peaks in its13C n.m.r. spectrum.
|
(CH3)2CHCH(CH3)COOH
|

